Stimuli-Responsive Nodal Dual-Drug Polymer Nanoparticles for Cancer Therapy
- PMID: 40292406
- PMCID: PMC12034347
- DOI: 10.2147/IJN.S517291
Stimuli-Responsive Nodal Dual-Drug Polymer Nanoparticles for Cancer Therapy
Abstract
Background: Polymeric drug delivery systems (DDSs) have gained significant attention in cancer therapy. However, these systems often respond to a single biological stimulus in tumor tissues or cells, limiting their effectiveness. While multi-sensitive DDSs improve therapeutic precision, their complex synthesis involving multi-step modifications remains challenging. Developing functionally integrated and simplified multiple stimuli-responsive DDSs is crucial to addressing tumor diversity and enhancing treatment efficacy.
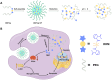
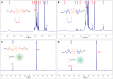
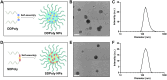
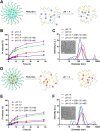
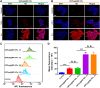
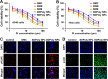
Methods and results: Here, we develop a dual-sensitive nodal dual-drug polymer nanoparticle (DDPoly NP) system for cancer therapy. This system combines a platinum(IV) prodrug (Cisplatin(IV)) with Demehylcantharidin (DMC) to create a dual-drug molecule (DDM). Then DDM is conjugated with methoxypolyethylene glycol (MPEG), forming a nodal dual-drug polymer (DDPoly). The amphiphilic polymer is capable of self-assembling into nanoparticles (DDPoly NPs) when in aqueous solution. The drug release experiments displayed that lower pH and reductive conditions simulating tumor microenvironment promoted the release of Cisplatin and DMC. Cytotoxicity studies demonstrated that DDPoly NPs exhibited superior anti-cancer activity compared to the single-drug system (SDPoly NPs). The IC50 values of DDPoly NPs against A549 cells (15.37 μM) and HeLa cells (17.05 μM) were significantly lower than those observed for SDPoly NPs, which were 40.48 μM for A549 cells and 38.11 μM for HeLa cells, respectively.
Conclusion: The study developed dual stimuli-responsive DDPoly NPs based on acid- and reduction-sensitive DDM, enabling tumor-specific activation without additional responsive components. DDPoly NPs triggered Pt(II) release via reduction and generated DMC through acid hydrolysis. The synergistic effect of DDPoly NPs lies in that DMC could inhibit the expression of serine/threonine protein phosphatase 2A (PP2A) and further elevate the expression of hyper-phosphorylated Akt (pAKt), thus blocking DNA repair to enhance Pt(II)-induced apoptosis. DDPoly NPs showed enhanced anti-cancer efficacy against cancer cells compared to SDPoly NPs, highlighting its potential for nanomedicine development.
Keywords: cancer therapy; chemotherapy; platinum drug; polymer nanoparticle; stimuli-responsive.
© 2025 Kuang et al.
Conflict of interest statement
The author(s) report no conflicts of interest in this work.
Figures

Similar articles
-
Light-activatable dual prodrug polymer nanoparticle for precise synergistic chemotherapy guided by drug-mediated computed tomography imaging.Acta Biomater. 2019 Aug;94:459-468. doi: 10.1016/j.actbio.2019.05.047. Epub 2019 May 22. Acta Biomater. 2019. PMID: 31128323
-
Biodegradable and pH-Responsive Acetalated Dextran (Ac-Dex) Nanoparticles for NIR Imaging and Controlled Delivery of a Platinum-Based Prodrug into Cancer Cells.Mol Pharm. 2019 May 6;16(5):2083-2094. doi: 10.1021/acs.molpharmaceut.9b00058. Epub 2019 Apr 1. Mol Pharm. 2019. PMID: 30901218
-
Dual-Sensitive Charge-Conversional Polymeric Prodrug for Efficient Codelivery of Demethylcantharidin and Doxorubicin.Biomacromolecules. 2016 Aug 8;17(8):2650-61. doi: 10.1021/acs.biomac.6b00705. Epub 2016 Jul 13. Biomacromolecules. 2016. PMID: 27384255
-
The Smart Dual-Stimuli Responsive Nanoparticles for Controlled Anti-Tumor Drug Release and Cancer Therapy.Anticancer Agents Med Chem. 2021;21(10):1202-1215. doi: 10.2174/1871520620666200924110418. Anticancer Agents Med Chem. 2021. PMID: 32972353 Review.
-
Nanoparticle-enabled In Situ drug potency activation for enhanced tumor-specific therapy.Eur J Pharm Sci. 2025 Feb 1;205:106989. doi: 10.1016/j.ejps.2024.106989. Epub 2024 Dec 14. Eur J Pharm Sci. 2025. PMID: 39675436 Review.
References
-
- Xiao H, Yan L, Dempsey EM, et al. Recent progress in polymer-based platinum drug delivery systems. Prog Polym Sci. 2018;87:70–106.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

