Advancements in Injectable Hydrogels for Controlled Insulin Delivery: A Comprehensive Review of the Design, Properties and Therapeutic Applications for Diabetes and Its Complications
- PMID: 40292663
- PMCID: PMC11944538
- DOI: 10.3390/polym17060780
Advancements in Injectable Hydrogels for Controlled Insulin Delivery: A Comprehensive Review of the Design, Properties and Therapeutic Applications for Diabetes and Its Complications
Abstract
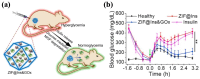
Glycemic management in diabetes patients remains heavily reliant on multiple daily insulin injections, which often leads to poor patient compliance and an elevated risk of hypoglycemia. To overcome these limitations, injectable hydrogels capable of encapsulating insulin within polymeric networks have emerged as a promising alternative. Ideally, a single injection can form an in situ depot that allows prolonged glycemic control and lower injection frequency. This review summarizes recent advances in injectable hydrogels for controlled insulin delivery, focusing on the polymer sources, crosslinking strategies, and stimuli-responsive release mechanisms. Synthetic polymers such as PEG, PNIPAM, and Pluronics dominate the current research due to their highly tunable properties, whereas naturally derived polysaccharides and proteins generally require further modifications for enhanced functionality. The crosslinking types, ranging from relatively weak physical interactions (hydrogen bonds, hydrophobic interactions, etc.) to dynamic covalent bonds with higher binding strength (e.g., Schiff base, phenylboronate ester), significantly influence the shear-thinning behavior and stimuli-responsiveness of hydrogel systems. Hydrogels' responsiveness to temperature, glucose, pH, and reactive oxygen species has enabled more precise insulin release, offering new options for improved diabetic management. Beyond glycemic regulation, this review also explores insulin-loaded hydrogels for treating complications. Despite the progress, challenges such as burst release, long-term biocompatibility, and scalability remain. Future research should focus on optimizing hydrogel design, supported by robust and comprehensive data.
Keywords: controlled insulin delivery; diabetes; diabetic complications; injectable hydrogels.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B.B., Stein C., Basit A., Chan J.C.N., Mbanya J.C., et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. - DOI - PMC - PubMed
-
- Roglic G. WHO Global report on diabetes: A summary. Int. J. Noncommun. Dis. 2016;1:3–8. doi: 10.4103/2468-8827.184853. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

