A Model of the Mechanisms Underpinning Unconventional Aqueous Humor Outflow
- PMID: 40293397
- PMCID: PMC12060054
- DOI: 10.1167/iovs.66.4.75
A Model of the Mechanisms Underpinning Unconventional Aqueous Humor Outflow
Abstract
Purpose: To develop a mathematical model of the unconventional outflow pathway.
Methods: The unconventional pathway is modeled as having two key components: the uveo-vortex and the trans-scleral pathways. The uveo-vortex pathway is modeled using Starling's law and the trans-scleral flow using predominately hydrostatic forces. We include transcytosis from the choriocapillaris (CC) and collapsibility of the suprachoroidal space (SCS) as particular features. There is considerable uncertainty in a number of model parameter values, and we identify the most significant ones using sensitivity analysis.
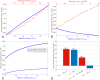
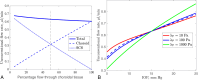
Results: The model successfully generates a fluid flow from anterior to posterior in the choroidal tissue and the SCS, which also demonstrates many of the known physiological features, including the insensitivity of the unconventional flow to fluctuations in the IOP, albumin removal by the trans-scleral flow, and the CC as a net absorber of fluid from, and supplier of albumin to, the choroidal tissue. The model supports the two previously proposed mechanisms of the action of prostaglandin F2α analogues.
Conclusions: We have developed a theoretical model of the unconventional aqueous outflow pathway that successfully captures its physiological features and elucidates the actions of prostaglandin F2α analogues and other drugs.
Conflict of interest statement
Disclosure:
Figures





References
-
- Nesterov AP. 13. The future for surgery in the glaucomas by increasing uveoscleral outflow. In: Cairns JE, ed. Glaucoma. New York: Grune & Stratton; 1986: 257–273.
-
- Bill A. The drainage of blood from the uvea and the elimination of aqueous humour in rabbits. Exp Eye Res. 1962; 1: 200–205. - PubMed
-
- Bill A. The aqueous humor drainage mechanism in the cynomolgus monkey (Macaca irus) with evidence for unconventional routes. Invest Ophthalmol Vis Sci. 1965; 4: 911–919. - PubMed
-
- Bill A, Hellsing K. Production and drainage of aqueous humor in the cynomolgus money (Macaca irus). Invest Ophthalmol Vis Sci. 1965; 4: 920–926. - PubMed
-
- Bill A. Conventional and uveo-scleral drainage of aqueous humour in the cynomolgus monkey (Macaca irus) at normal and high intraocular pressures. Exp Eye Res. 1966; 5(1): 45–54. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

