EDP-323, a First-In-Class, Once-Daily, Oral L-Protein Inhibitor for the Treatment of RSV: Results From a Phase 1 Study in Healthy Adults
- PMID: 40293399
- PMCID: PMC12036344
- DOI: 10.1111/cts.70231
EDP-323, a First-In-Class, Once-Daily, Oral L-Protein Inhibitor for the Treatment of RSV: Results From a Phase 1 Study in Healthy Adults
Abstract
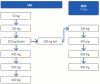
Respiratory syncytial virus (RSV) remains a significant health concern, particularly for vulnerable populations. Despite preventive strategies, there remains a need for effective antiviral treatments. EDP-323 is a first-in-class, potent oral selective non-nucleoside inhibitor of the large protein (L polymerase) of RSV under investigation for the treatment of RSV infection. This phase 1, randomized, double-blind, placebo-controlled study evaluated the safety and pharmacokinetics of EDP-323. This study included fasted single ascending dose (SAD; EDP-323 50/100/200/400/600/800 mg doses, 3:1 to placebo), fed multiple ascending dose (MAD; EDP-323200/400/600/800 mg doses, 3:1 to placebo), and food effect (EDP-323200 mg dose, 4:1 to placebo) cohorts in healthy adult participants. Key objectives were to assess the safety, tolerability, and pharmacokinetic (PK) profile of EDP-323 in plasma and urine, and to evaluate the effect of food intake on its pharmacokinetics. Among 82 randomized participants (SAD, n = 50; MAD, n = 32), EDP-323 was well tolerated up to the highest tested dose (800 mg once daily for 7 days). Adverse events (AEs) were reported in 14.6% of total participants, with the majority being mild and deemed unlikely related to the study drug. Headache was the most frequent AE (n = 3). PK analysis showed that EDP-323 was rapidly absorbed (Tmax = 3.0-5.0 h), with exposures increasing with ascending dose. The half-life of EDP-323 (t1/2 = 10.8-16.6 h) supported once-daily dosing, and no food effect was observed. EDP-323 demonstrated a favorable safety and PK profile, supporting its potential as a once-daily oral treatment for RSV.
Keywords: clinical trials; healthy subjects; infectious disease; oral; pharmacodynamics; pharmacokinetics; phase I; safety.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Medical writing assistance was provided by Shvetha Srinath, MSc, Katherine Stevens‐Favorite, PhD, and Brittany Eldridge, PhD, on behalf of Syneos Health, and supported by Enanta Pharmaceuticals.
All authors are currently employees of and hold stock in Enanta Pharmaceuticals Inc., Watertown, MA.
Figures


Similar articles
-
EDP-938, a Respiratory Syncytial Virus Inhibitor, in a Human Virus Challenge.N Engl J Med. 2022 Feb 17;386(7):655-666. doi: 10.1056/NEJMoa2108903. N Engl J Med. 2022. PMID: 35172056 Clinical Trial.
-
Pharmacokinetics of Zilurgisertib With and Without Food from Single and Multiple Ascending Dose Phase 1 Studies in Healthy Adults.Eur J Drug Metab Pharmacokinet. 2025 Jan;50(1):65-80. doi: 10.1007/s13318-024-00926-z. Epub 2024 Dec 9. Eur J Drug Metab Pharmacokinet. 2025. PMID: 39652202 Free PMC article. Clinical Trial.
-
A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects.CNS Drugs. 2018 Nov;32(11):1053-1067. doi: 10.1007/s40263-018-0578-5. CNS Drugs. 2018. PMID: 30374683 Free PMC article. Clinical Trial.
-
Pharmacokinetics of EDP-420 after ascending single oral doses in healthy adult volunteers.Antimicrob Agents Chemother. 2009 May;53(5):1786-92. doi: 10.1128/AAC.01270-08. Epub 2009 Feb 17. Antimicrob Agents Chemother. 2009. PMID: 19223626 Free PMC article. Clinical Trial.
-
Single- and multiple-ascending dose studies to evaluate the safety, tolerability, and pharmacokinetics of daclatasvir and asunaprevir in healthy male Japanese subjects.Int J Clin Pharmacol Ther. 2015 Apr;53(4):292-302. doi: 10.5414/CP202186. Int J Clin Pharmacol Ther. 2015. PMID: 25740262 Clinical Trial.
References
-
- Centers for Disease Control and Prevention , “Clinical Overview of RSV,” (2024), accessed September 9, 2024, https://www.cdc.gov/rsv/hcp/clinical‐overview/index.html.
-
- Centers for Disease Control and Prevention , “Surveillance of RSV,” (2024), accessed September 9, 2024, https://www.cdc.gov/rsv/php/surveillance/?CDC_AAref_Val=https://www.cdc.....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

