Dinitrogen Splitting without Barrier or Visible Light-Driven: Reactions of Vanadium Trimers and Dimers with Dinitrogen
- PMID: 40293441
- PMCID: PMC12144876
- DOI: 10.1002/chem.202500432
Dinitrogen Splitting without Barrier or Visible Light-Driven: Reactions of Vanadium Trimers and Dimers with Dinitrogen
Abstract
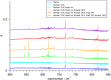
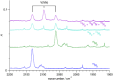
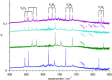
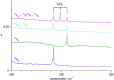
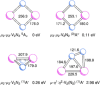
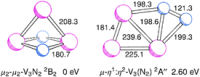
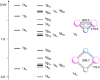
The reaction of V atoms and small clusters with dinitrogen has been investigated by matrix isolation in solid Ne and by complementary quantum chemical calculations. The experiments disclose striking differences between the reactivity of V atoms, dimers, and trimers toward dinitrogen. Whereas V atoms react only by formation of complexes, both V2 and V3 react by cleavage of the N2 triple bond to form nitride clusters. Whereas V3 reacts immediately at the conditions of matrix deposition, the reaction of V2 requires activation by irradiation with visible light. There is evidence for two isomers of V2N2, a planar one with a 5Au electronic term and a long V─V distance, and a folded one with a 1A1 term and a short V─V distance. Furthermore, there are also complexes of V2 and V3 with N2 that contain activated N2 units.
Keywords: cluster; density functional calculations; matrix isolation; nitrogen activation; vanadium.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
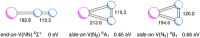
References
Grants and funding
LinkOut - more resources
Full Text Sources

