Molecular and Morphological Circuitry of the Octopus Sucker Ganglion
- PMID: 40293445
- PMCID: PMC12036646
- DOI: 10.1002/cne.70055
Molecular and Morphological Circuitry of the Octopus Sucker Ganglion
Abstract
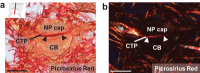
The octopus sucker is a profoundly complex sensorimotor structure. Each of the hundreds of suckers that line the octopus arm can move independently or in concert with one another. These suckers also contain an intricate sensory epithelium, enriched with chemotactile receptors. Much of the massive nervous system embedded in the octopus arm mediates control of the suckers. Each arm houses a large axial nerve cord (ANC), which features local enlargements corresponding to each sucker. There is also a sucker ganglion, a peripheral nervous element, situated in the stalk of every sucker. The structure and function of the sucker ganglion remain obscure. We examined the cellular organization and molecular composition of the sucker ganglion in Octopus bimaculoides. The sucker ganglion has an ellipsoid shape and features an unusual organization: the neuropil of the ganglion is distributed as a cap aborally (away from the sucker) and a small pocket orally (toward the sucker), with neuronal cell bodies concentrated in the space between. Using in situ hybridization, we detected positive expression of sensory (PIEZO) and motor (LHX3 and MNX) neuron markers in the sucker ganglion cell bodies. Nerve fibers spread out from the sucker ganglion, targeting the surrounding sucker musculature and the oral roots extending to the ANC. Our results indicate that the sucker ganglion is composed of both sensory and motor elements and suggest that this ganglion is not a simple relay for the ANC, but facilitates local reflexes for each sucker.
Keywords: Octopus bimaculoides; cephalopod; invertebrate; muscular hydrostat; neural circuitry; neuroethology; sensorimotor control.
© 2025 The Author(s). The Journal of Comparative Neurology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Update of
-
Molecular and morphological circuitry of the octopus sucker ganglion.bioRxiv [Preprint]. 2025 Feb 11:2025.02.10.637560. doi: 10.1101/2025.02.10.637560. bioRxiv. 2025. Update in: J Comp Neurol. 2025 May;533(5):e70055. doi: 10.1002/cne.70055. PMID: 39990388 Free PMC article. Updated. Preprint.
References
-
- Altman, J. 1968. “The Nervous Control of Arm and Buccal Movements in Octopus vulgaris.” Doctoral thesis, University College London.
-
- Altman, J. 1971. “Control of Accept and Reject Reflexes in the Octopus.” Nature 229: 204–206. - PubMed
-
- Arshadi, C. , Günther U., Eddison M., Harrington K. I. S., and Ferreira T. A. 2021. “SNT: A Unifying Toolbox for Quantification of Neuronal Anatomy.” Nature Methods 18: 374–377. - PubMed
-
- Baratte, S. , and Bonnaud L. 2009. “Evidence of Early Nervous Differentiation and Early Catecholaminergic Sensory System During Sepia officinalis Embryogenesis.” Journal of Comparative Neurology 517: 539–549. - PubMed
-
- Bidel, F. , Bennett N. C., and Wardill T. J. 2022. “ Octopus Bimaculoides' Arm Recruitment and Use During Visually Evoked Prey Capture.” Current Biology 32: 4727–4733.e3. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

