RhePort 1.3 enhances early identification of inflammatory rheumatic diseases: a prospective study in German rheumatology settings
- PMID: 40293492
- PMCID: PMC12037674
- DOI: 10.1007/s00296-025-05861-z
RhePort 1.3 enhances early identification of inflammatory rheumatic diseases: a prospective study in German rheumatology settings
Abstract
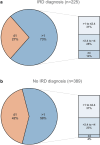
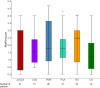
More efficient means of identifying patients with inflammatory rheumatic diseases (IRDs) could allow earlier diagnosis and treatment. The objective of this study was to evaluate the characteristics of a revised version of an online patient questionnaire-based self-referral tool, RhePort 1.3. This prospective study included adult patients with musculoskeletal complaints presenting for a first rheumatology visit at German RheumaDatenRhePort (RHADAR) rheumatology network centers. All patients completed the RhePort 1.3 questionnaire on patient characteristics and symptoms. Data from RhePort 1.3 were compared with historical data from previous versions. Of 614 patients, 225 (36.6%) were diagnosed with an IRD by a rheumatologist and 164/225 IRD patients (72.9%) had a RhePort 1.3 score > 1, the cut-off point used to determine the need for rheumatologic evaluation. A score > 1 was associated with an approximately two-fold higher IRD risk (odds ratio [95% confidence interval] of 1.98 [1.39, 2.83] vs ≤ 1) and had good sensitivity (73%) and moderate specificity (42%). Among patients referred through a standard referral pathway (n = 283), RhePort 1.3 scores > 1 in addition to physician referral were associated with increases in rheumatology-diagnosed IRD rates from 33.2% (physician referral only) to 45.7%. RhePort 1.3 had higher accuracy than earlier versions (54% vs 35%). We conclude that modest changes to the RhePort questionnaire resulted in increased accuracy. A score > 1 was associated with a doubled risk for an IRD and higher IRD rates in physician-referred patients. These data suggest that RhePort has the potential to streamline the rheumatologist's workload and improve resource use. Further modifications are required to improve specificity.
Keywords: Online system; Referral and consultation; Rheumatic diseases; Rheumatology; Surveys and questionnaires; Workload.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: C-BvdD received travel/meeting support from AbbVie and Galapagos/Alpha sigma. SK received grants from AbbVie, Novartis, and Sparrow, and consulting and/or speaker’s fees from AbbVie, Celgene, Chugai, Galapagos, Novartis, and Siemens Healthineers. ME received funding for the present study for data analysis from RHADAR GbR and also received consulting fees from AbbVie and RHADAR, speaker’s fees from AbbVie, Janssen-Cilag, Sanofi, and Swedish Orphan Biovitrum, and is on the advisory board of Chugai Pharma Germany. KK received speaker’s fees from AbbVie, Galapagos, Novartis, Rheumakademie, and UCB and travel/meeting support from UCB. GG received speaker’s fees from AbbVie, Galapagos, and Novartis and travel/meeting support from AbbVie and Novartis. JW received consulting and/or speaker’s fees from AbbVie, Janssen-Cilag, and Novartis. SS-M received speakers fees from AbbVie, Boehringer Ingelheim, Eli Lilly, GSK, Janssen-Cilag, Novartis, and UCB. MW received consulting and/or speaker’s fees from AbbVie, Fresenius, Galapagos, Lilly, and UCB and travel/meeting support from AbbVie, Galapagos, GSK, Lilly, and UCB. PB-B received speaker’s fees from AbbVie, Boehringer Ingelheim, Chugai/Roche, Janssen-Cilag, Novartis, Pfizer, and UCB and travel/meeting support from AbbVie. MR, PR, FS, CK, and WV have no conflicts of interest to disclose. C-BvdD, SK, KK, GG, SS-M, CK, WV, MW, and PB-B are members of RheumaDatenRhePort (RHADAR) GbR. Ethical approval Ethical approval for this study was obtained from the Ethics Committee of Ärztekammer Nordrhein (number 2021387; November 11, 2021) and the study was conducted in compliance with the Declaration of Helsinki. All patients enrolled in the study provided written informed consent.
Figures
References
-
- Gwinnutt JM, Symmons DPM, MacGregor AJ, Chipping JR, Marshall T, Lunt M et al (2017) Twenty-year outcome and association between early treatment and mortality and disability in an inception cohort of patients with rheumatoid arthritis: results from the Norfolk arthritis register. Arthritis Rheumatol 69(8):1566–1575. 10.1002/art.40090 - DOI - PMC - PubMed
-
- Albuquerque CP, Reis APMG, Vargas Santos AB, Bértolo MB, Júnior PL, Neubarth Giorgi RD et al (2023) Do it fast! Early access to specialized care improved long-term outcomes in rheumatoid arthritis: data from the REAL multicenter observational study. Adv Rheumatol 63(1):17. 10.1186/s42358-023-00301-7 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

