SPE-CZE-MS Quantifies Zeptomole Amounts of Phosphorylated Peptides
- PMID: 40293921
- PMCID: PMC12153072
- DOI: 10.1021/acs.jproteome.5c00194
SPE-CZE-MS Quantifies Zeptomole Amounts of Phosphorylated Peptides
Abstract
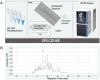
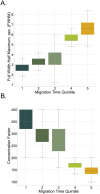
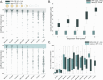
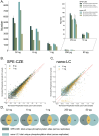
Capillary zone electrophoresis (CZE) is gaining attention in the field of single-cell proteomics for its ultralow-flow and high-resolution separation abilities. Even more sample-limited yet rich in biological information are phosphoproteomics experiments, as the phosphoproteome composes only a fraction of the whole cellular proteome. Rapid analysis, high sensitivity, and maximization of sample utilization are paramount for single-cell analysis. Some challenges of coupling CZE analysis with mass spectrometry analysis (MS) of complex mixtures include 1. sensitivity due to volume loading limitations of CZE and 2. incompatibility of MS duty cycles with electropherographic time scales. Here, we address these two challenges as applied to single-cell-equivalent phosphoproteomics experiments by interfacing a microchip-based CZE device integrated with a solid-phase-extraction (SPE) bed with the Orbitrap Astral mass spectrometer. Using 225 phosphorylated peptide standards and phosphorylated peptide-enriched mouse brain tissue, we investigate microchip-based SPE-CZE functionality, quantitative performance, and complementarity to nano-LC-MS (nLC-MS) analysis. We highlight unique SPE-CZE separation mechanisms that can empower fit-for-purpose applications in single-cell-equivalent phosphoproteomics.
Keywords: Astral; SPE; capillary zone electrophoresis; phosphoproteomics; preconcentration.
Figures

Update of
-
SPE-CZE-MS quantifies zeptomole concentrations of phosphorylated peptides.bioRxiv [Preprint]. 2024 Dec 14:2024.12.07.627347. doi: 10.1101/2024.12.07.627347. bioRxiv. 2024. Update in: J Proteome Res. 2025 Jun 6;24(6):3049-3061. doi: 10.1021/acs.jproteome.5c00194. PMID: 39713305 Free PMC article. Updated. Preprint.
References
-
- Cheng Y. F., Wu S., Chen D. Y., Dovichi N. J.. Interaction of capillary zone electrophoresis with a sheath flow cuvette detector. Anal. Chem. 1990;62:496–503. doi: 10.1021/ac00204a014. - DOI
-
- Horiuchi K., Dutta P.. Joule heating effects in electroosmotically driven microchannel flows. Int. J. Heat Mass Transfer. 2004;47:3085–3095. doi: 10.1016/j.ijheatmasstransfer.2004.02.020. - DOI
-
- Burgreen D., Nakache F. R.. Electrokinetic Flow in Ultrafine Capillary Slits 1. J. Phys. Chem. 1964;68(5):1084–1091. doi: 10.1021/j100787a019. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

