PLT012, a Humanized CD36-Blocking Antibody, Is Effective for Unleashing Antitumor Immunity Against Liver Cancer and Liver Metastasis
- PMID: 40294022
- PMCID: PMC7617665
- DOI: 10.1158/2159-8290.CD-24-1409
PLT012, a Humanized CD36-Blocking Antibody, Is Effective for Unleashing Antitumor Immunity Against Liver Cancer and Liver Metastasis
Abstract
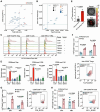
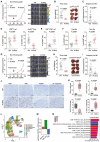
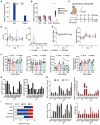
Tumor cells develop various strategies to evade immune surveillance, one of which involves altering the metabolic state of the tumor microenvironment. In response to metabolic stress in the tumor microenvironment, several tumor-infiltrating immune subsets upregulate CD36 to take up lipids. This leads to impaired antitumor immunity, as intratumoral regulatory T cells exhibit increased survival and suppressive activity, whereas CD8+ T cells become more susceptible to ferroptosis and exhaustion. In this study, we develop a humanized anti-CD36 IgG4 antibody, PLT012, against the lipid-binding domain of CD36 with excellent safety and favorable pharmacokinetic features in mice and cynomolgus monkeys. PLT012 alone or in combination with PD-L1 blockade or standard-of-care immunotherapy results in robust antitumor immunity in both immunotherapy-sensitive and -resistant hepatocellular carcinomas (HCC). Notably, PLT012 also reprograms the immune landscape of human HCC ex vivo. Our findings provide proof-of-concept evidence that PLT012 reprograms antitumor immunity in HCC, positioning it as a first-in-class immunotherapy targeting CD36.
Significance: Despite the success of cancer immunotherapies, like immune checkpoint inhibitors, many patients still fail to demonstrate significant responses because of metabolic constraints in tumors. PLT012 rejuvenates antitumor immunity by targeting metabolic pathways to reprogram the immune landscape of liver cancer and liver metastasis, with potential to influence future HCC immunotherapy.
©2025 American Association for Cancer Research.
Figures




References
MeSH terms
Substances
Grants and funding
- R01DK122813/National Institutes of Health (NIH)
- Helmut Horten Stiftung (Helmut Horten Foundation)
- Swiss Cancer Foundation (SCF)
- 310030L_208130/Swiss Science National Foundation
- MND-MAB-D-112095/Ministry of national defence-medical affair bureau
- R01 DK122813/DK/NIDDK NIH HHS/United States
- NSTC111-2314-B-182A-160-MY2/National Science and Technology Council (NSTC)
- MOE-113-YSFMN-1009-001-P1/Ministry of education in Taiwan
- Cancer Research Institute (CRI)
- 205930/swiss science national foundation
- 14104820/Hong Kong SAR
- 112-2628-B-016-002/National Science and Technology Council (NSTC)
- NSTC113-2314-B-182A-068/National Science and Technology Council (NSTC)
- SFU MED Research Promotion Fund
- 163204/SNSF_/Swiss National Science Foundation/Switzerland
- IZLCZ0_206083/Swiss Science National Foundation
- CRSII5_205930/Swiss Science National Foundation
- TMCG-3_213736/Swiss Science National Foundation
- 113-2628-B-016-005/National Science and Technology Council (NSTC)
- NSTC112-2628-B-182A-007-MY3/National Science and Technology Council (NSTC)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

