NF-κB signaling driven by oncogenic Ras contributes to tumorigenesis in a Drosophila carcinoma model
- PMID: 40294135
- PMCID: PMC12037074
- DOI: 10.1371/journal.pbio.3002663
NF-κB signaling driven by oncogenic Ras contributes to tumorigenesis in a Drosophila carcinoma model
Abstract
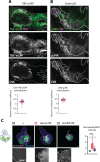
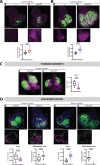
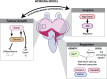
Cancer-driving mutations synergize with inflammatory stress signaling pathways during carcinogenesis. Drosophila melanogaster tumor models are increasingly recognized as models to inform conserved molecular mechanisms of tumorigenesis with both local and systemic effects of cancer. Although initial discoveries of the Toll-NFκB signaling pathway in development and immunity were pioneered in Drosophila, limited information is available for its role in cancer progression. Using a well-studied cooperative RasV12-driven epithelial-derived tumor model, we here describe functions of Toll-NF-κB signaling in malignant RasV12, scrib- tumors. The extracellular Toll pathway components ModSP and PGRP-SA and intracellular signaling Kinase, Pelle/IRAK, are rate-limiting for tumor growth. The Toll pathway NFκB protein Dorsal as well as cactus/IκΒ show elevated expression in tumors with highest expression in invasive cell populations. Oncogenic RasV12, and not loss of scribble, confers increased expression and heterogenous distribution of two Dorsal isoforms, DorsalA and DorsalB, in different tumor cell populations. Mechanistic analyses demonstrates that Dorsal, in concert with the BTB-transcription factor Chinmo, drives growth and malignancy by suppressing differentiation, counteracting apoptosis, and promoting invasion of RasV12, scrib- tumors.
Copyright: © 2025 Dillard et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Comment in
-
The innate immune system: A double-edged sword.PLoS Biol. 2025 Apr 29;23(4):e3003088. doi: 10.1371/journal.pbio.3003088. eCollection 2025 Apr. PLoS Biol. 2025. PMID: 40300148 Free PMC article.
References
-
- Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059 - DOI - PubMed
-
- Aggarwal BB, Sung B. NF-κB in cancer: a matter of life and death. Cancer Discov. 2011;1(6):469–71. doi: 10.1158/2159-8290.CD-11-0260 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

