Validity and Reliability of Clinical and Patient-Reported Outcomes in Multisystem Proteinopathy 1
- PMID: 40294152
- PMCID: PMC12257134
- DOI: 10.1002/acn3.70064
Validity and Reliability of Clinical and Patient-Reported Outcomes in Multisystem Proteinopathy 1
Abstract
Objective: Valosin-containing protein (VCP)-associated multisystem proteinopathy 1 (MSP1) is caused by variants in the VCP gene. MSP1 results in various phenotypes including progressive myopathy, Paget's disease of bone, frontotemporal dementia, amyotrophic lateral sclerosis, and parkinsonism, among others. Our study aimed to validate functional clinical outcome assessments (COA) and patient-reported outcomes (PRO) to inform clinical care practices and future clinical trial design. In addition, we evaluated the test-retest reliability of these COAs within clinics and remote environments.
Methods: Patients completed a battery of COA and PRO across a 2-day traditional onsite visit and a 2-day remote visit within their home environment. All COA and PRO deemed safe and feasible to complete based on participants' level of function and/or home environment were collected at each visit.
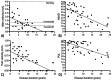
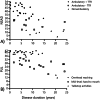
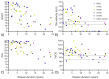
Results: Forty-six total patients enrolled in our study, 34 in our full study and 12 in an expanded remote-only cohort. Functional COA measured decline over reported disease duration in this cross-sectional group and significantly correlated with PRO (rho > 0.5, p < 0.001). Differences in lower and upper extremity involvement were noted across variant groups. Performance of functional COA was reliable and safe within and across onsite and remote testing environments (ICC > 0.7, p < 0.001).
Interpretation: Functional COA and PRO are valid and reliable to measure abilities in participants with MSP1. Testing can be completed reliably within the home, which could expand equitable access to clinical care and/or future clinical trial participation. Prospective longitudinal data collection is ongoing to understand outcome sensitivity and meaningful change over time.
Keywords: VCP‐associated multisystem proteinopathy; function; natural history.
© 2025 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Esteller D., Schiava M., Verdu‐Diaz J., et al., “Analysis of Muscle Magnetic Resonance Imaging of a Large Cohort of Patient With VCP‐Mediated Disease Reveals Characteristic Features Useful for Diagnosis,” Journal of Neurology 270, no. 12 (2023): 5849–5865, 10.1007/s00415-023-11862-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

