Dysregulation of MYBL2 impairs extravillous trophoblast lineage development and function, contributing to recurrent spontaneous abortion
- PMID: 40294258
- PMCID: PMC12067203
- DOI: 10.1073/pnas.2421653122
Dysregulation of MYBL2 impairs extravillous trophoblast lineage development and function, contributing to recurrent spontaneous abortion
Abstract
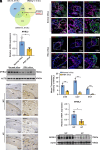
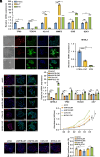
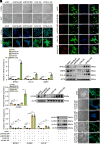
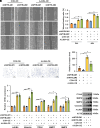
Recurrent spontaneous abortion (RSA) is a pregnancy-related condition characterized by a complex etiology. While placental trophoblast dysfunction is strongly associated with the development and progression of RSA, the underlying molecular mechanisms remain poorly understood. In this study, we observed a significant decrease in the expression of MYB Proto-Oncogene Like 2 (MYBL2) in the villous tissue of patients with RSA and the placentas of abortion-prone (AP) mice. Utilizing human trophoblast stem cells (hTSCs), we identified MYBL2 as a critical regulator of hTSCs stemness maintenance, promoting the expression of the stemness-associated genes Tumor protein p63 (TP63) and TEA Domain Transcription Factor 4 (TEAD4). Furthermore, MYBL2 facilitates the differentiation of hTSCs into extravillous trophoblast (EVT) by positively regulating Ajuba LIM Protein (AJUBA) expression. Using HTR-8/SVneo cell line, an immortalized EVT-like model, we found that MYBL2 positively regulates AJUBA expression by binding to the distal region of the AJUBA promoter. Additionally, the MYBL2-AJUBA axis enhances the migration and invasion of HTR-8/SVneo cells by suppressing the Hippo signaling pathway. Our study indicates that the dysregulation of MYBL2 expression in placental trophoblasts is associated with the pathogenesis of RSA, highlighting its potential as a therapeutic target for this condition.
Keywords: Hippo; MYBL2; placenta; recurrent spontaneous abortion; trophoblast cell.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
-
- Dimitriadis E., Menkhorst E., Saito S., Kutteh W. H., Brosens J. J., Recurrent pregnancy loss. Nat. Rev. Dis. Primers. 6, 98 (2020). - PubMed
-
- Burton G. J., Jauniaux E., Charnock-Jones D. S., The influence of the intrauterine environment on human placental development. Int. J. Dev. Biol. 54, 303–312 (2010). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

