Dopamine induces fear extinction by activating the reward-responding amygdala neurons
- PMID: 40294263
- PMCID: PMC12067255
- DOI: 10.1073/pnas.2501331122
Dopamine induces fear extinction by activating the reward-responding amygdala neurons
Abstract
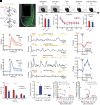
The extinction of conditioned fear responses is crucial for adaptive behavior, and its impairment is a hallmark of anxiety disorders such as posttraumatic stress disorder. Fear extinction takes place when animals form a new memory that suppresses the original fear memory. In the case of context-dependent fear memory, the new memory is formed within the reward-responding posterior subset of basolateral amygdala (BLA) that is genetically marked by Ppp1r1b+ neurons. These memory engram cells suppress the activity of the original fear-responding Rspo2+ engram cells present in the anterior BLA, hence fear extinction. However, the neurological nature of the teaching signal that instructs the formation of fear extinction memory in the Ppp1r1b+ neurons is unknown. Here, we demonstrate that ventral tegmental area (VTA) dopaminergic signaling drives fear extinction in distinct BLA neuronal populations. We show that BLA fear and extinction neuronal populations receive topographically divergent inputs from VTA dopaminergic neurons via differentially expressed dopamine receptors. Fiber photometry recordings of dopaminergic activity in the BLA reveal that dopamine (DA) activity is time-locked to freezing cessation in BLA fear extinction neurons, but not BLA fear neurons. Furthermore, this dopaminergic activity in BLA fear extinction neurons correlates with extinction learning. Finally, using projection-specific optogenetic manipulation, we find that activation of the VTA DA projections to BLA reward and fear neurons accelerated or impaired fear extinction, respectively. Together, this work demonstrates that dopaminergic activity bidirectionally controls fear extinction by distinct patterns of activity at BLA fear and extinction neurons.
Keywords: amygdala; dopamine; fear extinction; reward; valence.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures





References
-
- Shalev A., Liberzon I., Marmar C., Post-traumatic stress disorder. N. Engl. J. Med. 376, 2459–2469 (2017). - PubMed
-
- Pavlov I. P., Conditioned Reflexes (Oxford University Press, 1927).
-
- Zhang X., Kim J., Tonegawa S., Amygdala reward neurons form and store fear extinction memory. Neuron 105, 1077–1093.e7 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

