Hematopoietic Stem Cell Transplantation Outcomes for High-Risk AML: A Report From the Children's Oncology Group
- PMID: 40294366
- PMCID: PMC12140909
- DOI: 10.1200/JCO-24-01841
Hematopoietic Stem Cell Transplantation Outcomes for High-Risk AML: A Report From the Children's Oncology Group
Erratum in
-
Erratum: Hematopoietic Stem Cell Transplantation Outcomes for High-Risk AML: A Report From the Children's Oncology Group.J Clin Oncol. 2025 Aug 20;43(24):2757. doi: 10.1200/JCO-25-01516. Epub 2025 Jul 14. J Clin Oncol. 2025. PMID: 40658917 No abstract available.
Abstract
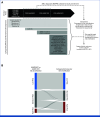
Purpose: Hematopoietic stem cell transplantation (HSCT) is used as consolidation for pediatric patients with high-risk AML in first complete remission (CR1). The definition of high-risk AML has evolved considerably over the past two decades with the successive identification of new unfavorable risk factors. We conducted a cross-study analysis to determine whether HSCT improves the outcomes of patients with contemporarily defined high-risk AML.
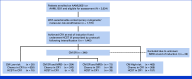
Methods: We combined data from AAML0531 and AAML1031, the last two phase III clinical trials completed by the Children's Oncology Group (COG). These two trials established the prognostic importance of measurable residual disease (MRD) and several high-risk cryptic cytogenetic/molecular (CM) alterations, which were applied to reclassify patients in the current COG phase III clinical trial, AAML1831. We compared the outcomes after HSCT in CR1 with those after chemotherapy alone in CR1 in the redefined high-risk group.
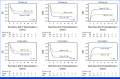
Results: Our study cohort comprised 463 patients with high-risk CM alterations and 72 patients with standard-risk (SR) CM results with positive MRD at end of induction I. In all, 33.9% and 45.8% of these groups underwent HSCT in CR1, respectively. HSCT was associated with decreased relapse and improved disease-free survival (DFS) in both groups. In the high-risk CM group, 5-year DFS was 26.0% (95% CI, 20.6 to 31.6) and 49.8% (95% CI, 41.7 to 57.4; P < .001) in patients receiving chemotherapy alone and HSCT, respectively. In the SR CM and MRD+ groups, DFS was 16.9% (95% CI, 4.3 to 36.7) compared with 50.9% (95% CI, 32.7 to 66.5; P = .032). HSCT was also associated with improvement in outcomes based on multivariable analysis and across subgroups defined by clinical trial and by high-risk CM subtype, with the exception of chromosome 7 or 5 loss.
Conclusion: HSCT was associated with improved outcomes in pediatric patients with contemporarily defined high-risk AML.
Trial registration: ClinicalTrials.gov NCT00372593 NCT01371981.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol. 2014;32:3021–3032. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

