Extended Caffeine for Apnea in Moderately Preterm Infants: The MoCHA Randomized Clinical Trial
- PMID: 40294395
- PMCID: PMC12038711
- DOI: 10.1001/jama.2025.5791
Extended Caffeine for Apnea in Moderately Preterm Infants: The MoCHA Randomized Clinical Trial
Abstract
Importance: Hospitalization of moderately preterm infants may be prolonged while waiting for apnea of prematurity to resolve after discontinuing caffeine.
Objective: To evaluate whether extending caffeine treatment reduces the duration of hospitalization.
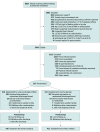
Design, setting, and participants: From February 2019 to December 2022, this randomized clinical trial in 29 US hospitals enrolled infants born at 29 to 33 weeks' gestation who at 33 to 35 weeks' postmenstrual age were receiving caffeine treatment with plans to discontinue it plus receiving full feeds (≥120 mL/kg/d). Follow-up was completed on March 20, 2023.
Interventions: Infants were randomized to oral caffeine citrate (10 mg/kg/d) or placebo until 28 days after discharge.
Main outcomes and measures: The primary outcome was days to discharge after randomization. Secondary outcomes included days to physiological maturity (apnea free for 5 consecutive days, receiving full oral feeds, and out of the incubator for at least 48 hours), postmenstrual age at discharge, all-cause hospital readmissions, all-cause sick and emergency department visits, safety outcomes, and death.
Results: A total of 827 infants (median gestational age, 31 weeks; 414 female [51%]) were randomized (416, caffeine; 411, placebo) out of the 878 planned before reaching the prespecified futility threshold. Days of hospitalization after randomization did not differ between groups (18.0 days [IQR, 10 to 30 days] for caffeine vs 16.5 [IQR, 10 to 27 days] for placebo; adjusted median difference, 0 days [95% CI, -1.7 to 1.7 days]), nor did days to physiological maturity differ (14.0 vs 15.0 days, adjusted median difference, -1 day [95% CI, -2.4 to 0.4 days]). Infants receiving caffeine were apnea free sooner (6.0 vs 10.0 days; adjusted median difference, -2.7 days [95% CI, -3.4 to -2.0 days ]) but had similar days to full oral feeding (7.5 vs 6.0 days, adjusted median difference, 0 days [95% CI, -0.1 to 0.1]). Rates of readmissions and sick visits did not differ between groups. There was no statistically significant difference in adverse events between the 2 groups.
Conclusions and relevance: In moderately preterm infants, continuation of caffeine treatment compared with placebo did not shorten hospitalization.
Trial registration: ClinicalTrials.gov Identifier: NCT03340727.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HD027871/HD/NICHD NIH HHS/United States
- U10 HD053119/HD/NICHD NIH HHS/United States
- UG1 HD021364/HD/NICHD NIH HHS/United States
- U10 HD021373/HD/NICHD NIH HHS/United States
- UG1 HD021385/HD/NICHD NIH HHS/United States
- UG1 HD027851/HD/NICHD NIH HHS/United States
- UG1 HD027853/HD/NICHD NIH HHS/United States
- UG1 HD027856/HD/NICHD NIH HHS/United States
- UG1 HD027880/HD/NICHD NIH HHS/United States
- UG1 HD027904/HD/NICHD NIH HHS/United States
- UG1 HD034216/HD/NICHD NIH HHS/United States
- UG1 HD040492/HD/NICHD NIH HHS/United States
- UG1 HD040689/HD/NICHD NIH HHS/United States
- UG1 HD053089/HD/NICHD NIH HHS/United States
- UG1 HD053109/HD/NICHD NIH HHS/United States
- UG1 HD068244/HD/NICHD NIH HHS/United States
- UG1 HD068270/HD/NICHD NIH HHS/United States
- UG1 HD068278/HD/NICHD NIH HHS/United States
- UG1 HD068263/HD/NICHD NIH HHS/United States
- UG1 HD068284/HD/NICHD NIH HHS/United States
- UG1 HD087226/HD/NICHD NIH HHS/United States
- UG1 HD087229/HD/NICHD NIH HHS/United States
- UL1 TR001117/TR/NCATS NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- UL1 TR000442/TR/NCATS NIH HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
- UL1 TR000042/TR/NCATS NIH HHS/United States
- UL1 TR000041/TR/NCATS NIH HHS/United States
- UL1 TR000006/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

