Dietary resistant starch protects against post-antibiotic intestinal damage by restoring microbial homeostasis and preserving intestinal barrier function in meat duck
- PMID: 40294558
- PMCID: PMC12059379
- DOI: 10.1016/j.psj.2025.105213
Dietary resistant starch protects against post-antibiotic intestinal damage by restoring microbial homeostasis and preserving intestinal barrier function in meat duck
Abstract
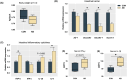
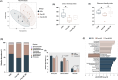
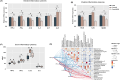
Resistant starch (RS) is recognized as a nutritional strategy that supports gut and overall host health by modulating gut microbiota. To directly assess the effects of RS on gut microbiota and its role in improving intestinal barrier function in meat ducks, this study first established an antibiotic-induced microbial dysbiosis model, which was characterized by reduced gut microbial diversity, intestinal dysfunction, and an inflammatory outburst following antibiotic exposure. Whereafter, in addition to the control group, ducks treated with antibiotics for 7 consecutive days were further allocated to two groups and fed the basal diet and RS diet that derived from 12 % raw potato starch until 21 d. The results demonstrated that dietary RS supplementation reversed the antibiotic-induced reduction in microbial diversity and restored the Firmicutes-to-Bacteroidetes ratio. Additionally, RS inclusion enriched beneficial bacterial genera, including Coprobacter, Odoribacter, and Faecalibacterium (LDA score > 3). Post-antibiotic intervention led to a reduction in villus density and muscular thickness, accompanied by a significant downregulation (P < 0.05) of zonula occludens-1 and mucin-2 expression, along with increased serum pro-inflammatory cytokine levels (P < 0.05). Notably, dietary RS supplementation significantly enhanced (P < 0.05) the expression of glucagon-like peptide receptor and the anti-apoptotic factor Bcl-2, while suppressing caspase transcription. This resulted in increased villus height and muscular thickness in the ileum (P < 0.05). Furthermore, RS intervention remarkably reduced (P < 0.05) pro-inflammatory cytokine levels, particularly interleukin-1β and tumor necrosis factor-α, in both the ileum and serum. These effects were likely linked to alterations in cecal microbiota, including increased abundances of Barnesiella, Ruminiclostridium 9, Megamonas, Faecalitalea, Adlercreutzia, Coprobacter and Collinsella. In conclusion, dietary RS supplementation mitigated antibiotic-induced cecal microbial dysbiosis and restored intestinal structure by promoting enterocyte proliferation and reducing apoptosis. Consequently, RS supplementation helped alleviate systemic inflammation in meat ducks following antibiotic treatment.
Keywords: Antibiotic; Gut microbiota; Inflammation; Meat ducks; Resistant starch.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Figures



Similar articles
-
The protective effects of dietary resistant starch against post-antibiotic bone loss in meat ducks associated with the recovery of caecal microbiota dysbiosis.Poult Sci. 2025 Aug;104(8):105238. doi: 10.1016/j.psj.2025.105238. Epub 2025 May 1. Poult Sci. 2025. PMID: 40424882 Free PMC article.
-
Astaxanthin supplementation enhances carcass performance, meat quality, and intestinal barrier function by modulating cecal microbiota and metabolomics in overfed Pekin ducks.Poult Sci. 2025 Aug;104(8):105310. doi: 10.1016/j.psj.2025.105310. Epub 2025 May 16. Poult Sci. 2025. PMID: 40441115 Free PMC article.
-
Dietary curcumin supplementation enhances growth performance and anti-inflammatory functions by modulating gut microbiota, microbiota-derived metabolites, and expression of inflammation-related genes in broilers.J Anim Sci. 2024 Jan 3;102:skae296. doi: 10.1093/jas/skae296. J Anim Sci. 2024. PMID: 39324614
-
Efficacy of Bacillus subtilis probiotic in preventing necrotic enteritis in broilers: a systematic review and meta-analysis.Avian Pathol. 2024 Dec;53(6):451-466. doi: 10.1080/03079457.2024.2359596. Epub 2024 Jul 3. Avian Pathol. 2024. PMID: 38776185
-
Systematic review on microbiome-related nutritional interventions interfering with the colonization of foodborne pathogens in broiler gut to prevent contamination of poultry meat.Poult Sci. 2024 May;103(5):103607. doi: 10.1016/j.psj.2024.103607. Epub 2024 Mar 5. Poult Sci. 2024. PMID: 38493536 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

