Effect of HPV integration on prognosis of young women with CIN2 in China: protocol for a multicentre prospective cohort study
- PMID: 40295124
- PMCID: PMC12039012
- DOI: 10.1136/bmjopen-2024-093863
Effect of HPV integration on prognosis of young women with CIN2 in China: protocol for a multicentre prospective cohort study
Abstract
Introduction: Cervical cancer, a major global health concern, is primarily caused by human papillomavirus (HPV) infection. Although cervical intraepithelial neoplasia grade 2 (CIN2), a precancerous lesion, exhibits high spontaneous regression rates (50%-60%), particularly in younger women, current clinical management lacks accurate risk stratification. This study examines HPV integration status as a prognostic biomarker in women aged 18-45 diagnosed with CIN2, with the objective of developing a predictive tool for personalised therapeutic strategies and minimising overtreatment in this high-regression population.
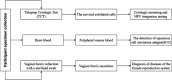
Method and analysis: This multicentre cohort study will be implemented across 20 tertiary Grade A hospitals in China, encompassing eastern, western, central and northern regions. It will recruit 240 CIN2 patients, collecting sociodemographic, lifestyle and medical history data via questionnaires. Clinical examinations will be performed at baseline and follow-up. Disease regression ((to cervical intraepithelial neoplasia grade 1 [CIN1] or lower)) and non-regression (persistent CIN2 or progression) will be evaluated. Prognostic factors will be analysed using Cox proportional hazards models, adjusting for confounders such as age, weight and socioeconomic status.
Ethics and dissemination: The cohort study protocol and informed consent procedures adhere to the Declaration of Helsinki and pertinent Chinese clinical research regulations. Ethical approval has been obtained from the Clinical Research Review Committee of the Fujian Maternal and Child Health Hospital (2022KYLLR01018) and from the participating hospitals. Written informed consent is secured from all participants prior to enrolment, with detailed information provided regarding study objectives, procedures, potential risks and benefits and participants' rights. Results will be published in peer-reviewed scientific journals, presented at academic meetings and conferences and released to the public through press releases.
Trial registration number: ClinicalTrials.gov (NCT05282095); Pre-results.
Keywords: EPIDEMIOLOGIC STUDIES; GYNAECOLOGY; HPV Infection; Human Papillomavirus Viruses.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Human papilloma virus testing in patient follow-up post cone biopsy due to high-grade cervical intraepithelial neoplasia.Gynecol Oncol. 2003 Mar;88(3):345-50. doi: 10.1016/s0090-8258(02)00137-3. Gynecol Oncol. 2003. PMID: 12648585
-
Performance of HPV E4 and p16INK4a biomarkers in predicting regression of cervical intraepithelial neoplasia grade 2 (CIN2): protocol for a historical cohort study.BMJ Open. 2022 Jul 6;12(7):e059593. doi: 10.1136/bmjopen-2021-059593. BMJ Open. 2022. PMID: 35793925 Free PMC article.
-
Role of FAM19A4/miR124-2 methylation analysis in predicting regression or non-regression of CIN2/3 lesions: a protocol of an observational longitudinal cohort study.BMJ Open. 2019 Jul 9;9(7):e029017. doi: 10.1136/bmjopen-2019-029017. BMJ Open. 2019. PMID: 31289088 Free PMC article.
-
Prevalence of Human Papillomavirus Genotypes Among Women With High-Grade Cervical Lesions in Beijing, China.Medicine (Baltimore). 2016 Jan;95(3):e2555. doi: 10.1097/MD.0000000000002555. Medicine (Baltimore). 2016. PMID: 26817906 Free PMC article.
-
Potential impact of combined high- and low-risk human papillomavirus infection on the progression of cervical intraepithelial neoplasia 2.J Obstet Gynaecol Res. 2014 Feb;40(2):561-9. doi: 10.1111/jog.12202. Epub 2013 Oct 22. J Obstet Gynaecol Res. 2014. PMID: 24147758