Population Pharmacokinetics of Rifampicin in Plasma and Cerebrospinal Fluid in Adults With Tuberculosis Meningitis
- PMID: 40295169
- PMCID: PMC12349950
- DOI: 10.1093/infdis/jiaf178
Population Pharmacokinetics of Rifampicin in Plasma and Cerebrospinal Fluid in Adults With Tuberculosis Meningitis
Abstract
Background: Several ongoing clinical trials are evaluating high-dose rifampicin (up to 35 mg/kg) for tuberculous meningitis (TBM). However, rifampicin pharmacokinetics at higher doses is not fully characterized, particularly in cerebrospinal fluid (CSF), the site of TBM disease.
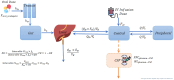
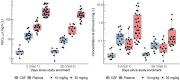
Methods: In a randomized controlled trial, adults with HIV-associated TBM were assigned to experimental arms of high-dose rifampicin (oral, 35 mg/kg; intravenous, 20 mg/kg) plus linezolid, with or without aspirin, or a control arm that received the standard of care with 10 mg/kg of oral rifampicin. Rifampicin concentrations, including the unbound fraction, were measured on plasma samples, and CSF was collected on days 3 and 28 of study enrollment. Data were analyzed by nonlinear mixed effects modeling.
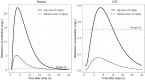
Results: In total, 400 plasma and 44 CSF rifampicin concentrations from 48 participants were used for model development. The median (range) age and weight were 39 years (25-78) and 60 kg (30-107). Rifampicin pharmacokinetics was best described by a 2-compartment disposition model with first-order transit oral absorption and elimination via saturable hepatic extraction. Typical clearance values for the standard dose for days 3 and 28 were 33.1 and 41.4 L/h, respectively; high-dose values were 46.1 and 70.2 L/h. The CSF-plasma ratio was approximately 6% and the equilibration half-life was 3.2 hours. Simulated standard-dose rifampicin did not reach CSF concentrations above the critical concentration for Mycobacterium tuberculosis.
Conclusions: CSF penetration with standard-dose rifampicin is low. Our findings support continued evaluation of high-dose rifampicin for TBM treatment.
Keywords: cerebrospinal fluid; high-dose rifampicin; modeling and simulation; population pharmacokinetics; tuberculosis meningitis.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts.
Figures
References
-
- van Toorn R, Schaaf HS, Laubscher JA, van Elsland SL, Donald PR, Schoeman JF. Short intensified treatment in children with drug-susceptible tuberculous meningitis. Pediatr Infect Dis J 2014; 33:248–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 203135/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- 214321/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- Bill & Melinda Gates Foundation
- U01 AI068632/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- K43TW011421/NH/NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- AI068632/MH/NIMH NIH HHS/United States
- 175479/UCL Wellcome Trust
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
- U01 AI170426/AI/NIAID NIH HHS/United States
- U01AI170426/NH/NIH HHS/United States
- AI106701/NH/NIH HHS/United States
- National Institute of Allergy and Infectious Diseases
- AI068634/NH/NIH HHS/United States
- Francis Crick Institute
- UM1 AI068632/AI/NIAID NIH HHS/United States
- Research Centre of Imperial College NHS Trust
- CC2112/WT_/Wellcome Trust/United Kingdom
- Infant Maternal Pediatric Adolescent AIDS Clinical Trials Group
- 64787/National Research Foundation
- South African Research Chairs Initiative of the Department of Science and Technology
- University of Cape Town Clinical PK Laboratory
- U01 AI068632/NH/NIH HHS/United States
- AI068636/NH/NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- CC2112/CRUK_/Cancer Research UK/United Kingdom
- K43 TW011421/TW/FIC NIH HHS/United States
- Adult Clinical Trial Group
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources

