Reproxalap in patients with seasonal allergic conjunctivitis: a systematic review and meta-analysis
- PMID: 40295385
- PMCID: PMC12037956
- DOI: 10.1186/s12348-025-00497-3
Reproxalap in patients with seasonal allergic conjunctivitis: a systematic review and meta-analysis
Abstract
Introduction: Seasonal allergic conjunctivitis (SAC) is a hypersensitivity condition characterized by itching, tearing, and redness. It affects over 20% of the general population with limited therapeutic options. Reproxalap, a novel small-molecule aldehyde trap, has emerged as a potential treatment option for SAC by targeting reactive aldehydes involved in inflammation. In this systematic review and meta-analysis, we assessed the efficacy and safety of Reproxalap in treating SAC.
Methods: Multiple databases were searched including PubMed, Cochrane Library, Scopus, and Google Scholar, to identify relevant studies. Clinical trials involving patients diagnosed with SAC and treated with Reproxalap (0.25% or 0.5%) were included. The primary outcomes were symptom relief (efficacy) and side effects (safety). Data extraction and risk of bias assessment were performed independently by two reviewers. Meta-analysis was conducted using RevMan 5.4 software.
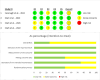
Results: Five RCTs involving 625 participants were included. Reproxalap significantly reduced ocular itching compared to control groups for both 0.25% (SMD: -0.31, 95% CI: -0.50 to -0.13, P = .001) and 0.5% concentrations (SMD: -0.31, 95% CI: -0.51 to -0.10, P = 0.004). No significant difference was observed between the two doses (SMD: -0.02, 95% CI: -0.23 to 0.19, P = 0.83). Side effects were more frequent in both Reproxalap groups compared to controls, with odds ratios of 35.31 (95% CI: 17.83 to 69.90, P < 0.00001) for 0.25% and 45.64 (95% CI: 18.49 to 112.66, P < 0.00001) for 0.5%. The 0.5% dose had significantly higher odds of side effects compared to the 0.25% dose (OR: 1.66, 95% CI: 1.10 to 2.51, P = 0.02). Heterogeneity was low for all outcomes (I2 = 0%).
Conclusion: Reproxalap reduces ocular itching associated with SAC. While both 0.25% and 0.5% concentrations are effective, safe and tolerable. Further research should focus on the long-term benefits and applicability of Reproxalap on diverse populations.
Keywords: Meta-analysis; Ocular itching; Reactive aldehyde species; Reproxalap; Seasonal allergic conjunctivitis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval was waived as this was a secondary analysis of online data. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Bielory BP, O’Brien TP, Bielory L. Management of seasonal allergic conjunctivitis: guide to therapy. Acta Ophthalmologica . 2012;90(5):399–407. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1755-3768.2011.02272.x. Cited 2024 Nov 9 - PubMed
-
- Ono SJ, Abelson MB. Allergic conjunctivitis: Update on pathophysiology and prospects for future treatment. Journal of Allergy and Clinical Immunology. 2005;115(1):118–22. Available from: https://www.jacionline.org/article/S0091-6749(04)03032-5/fulltext. Cited 2024 Nov 9 - PubMed
-
- Bielory L (2012) Allergic conjunctivitis: the evolution of therapeutic options. Allergy Asthma Proc 33(2):129–139 - PubMed
-
- Baab S, Le PH, Gurnani B, Kinzer EE. Allergic Conjunctivitis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. Available from: http://www.ncbi.nlm.nih.gov/books/NBK448118/. Cited 2024 Oct 31 - PubMed
-
- Pitt AD, Smith AF, Lindsell L, Voon LW, Rose PW, Bron AJ (2004) Economic and quality-of-life impact of seasonal allergic conjunctivitis in Oxfordshire. Ophthalmic Epidemiol 11(1):17–33 - PubMed
Publication types
LinkOut - more resources
Full Text Sources

