Development of viral infectious clones and their applications based on yeast and bacterial artificial chromosome platforms
- PMID: 40295404
- PMCID: PMC12037452
- DOI: 10.1186/s43556-025-00266-7
Development of viral infectious clones and their applications based on yeast and bacterial artificial chromosome platforms
Abstract
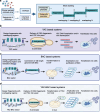
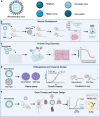
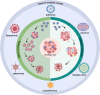
Infectious Clones represent a foundational technique in the field of reverse genetics, allowing for the construction and manipulation of full-length viral genomes. The main methods currently used for constructing viral infectious clones include Transformation-associated recombination (TAR), which is based on Yeast Artificial Chromosome (YAC) and Bacterial Artificial Chromosome (BAC). The YAC and BAC systems are powerful tools that enable the clones and manipulation of large DNA fragments, making them well-suited for the construction of full-length viral genomes. These methods have been successfully applied to construct infectious clones for a wide range of viruses, including coronaviruses, herpesviruses, flaviviruses and baculoviruses. The rescued recombinant viruses from these infectious clones have been widely used in various research areas, such as vaccine development, antiviral drug screening, pathogenesis and virulence studies, gene therapy and vector design. However, as different viruses possess unique biological characteristics, the challenge remains in how to rapidly obtain infectious clones for future research. In summary, this review introduced the development and applications of infectious clones, with a focus on the YAC, BAC and combined YAC-BAC technologies. We emphasize the importance of these platforms in various research areas and aim to provide deeper insights that can advance the platform and broaden its application horizons.
Keywords: Application; Bacterial artificial chromosome (BAC); Reverse genetics; Virus Infectious Clones; Yeast artificial chromosome (YAC).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no conflicts of interest.
Figures
Similar articles
-
Construction of human chromosome 16- and 5-specific circular YAC/BAC libraries by in vivo recombination in yeast (TAR cloning).Genomics. 1998 Oct 1;53(1):21-8. doi: 10.1006/geno.1998.5469. Genomics. 1998. PMID: 9787074
-
Large-insert BAC/YAC libraries for selective re-isolation of genomic regions by homologous recombination in yeast.Genomics. 2001 Sep;77(1-2):27-34. doi: 10.1006/geno.2001.6616. Genomics. 2001. PMID: 11543629
-
Construction of a full-length infectious bacterial artificial chromosome clone of duck enteritis virus vaccine strain.Virol J. 2013 Nov 6;10:328. doi: 10.1186/1743-422X-10-328. Virol J. 2013. PMID: 24195756 Free PMC article.
-
Bacterial-Artificial-Chromosome-Based Genome Editing Methods and the Applications in Herpesvirus Research.Microorganisms. 2023 Feb 26;11(3):589. doi: 10.3390/microorganisms11030589. Microorganisms. 2023. PMID: 36985163 Free PMC article. Review.
-
[BAC system: A novel method for manipulation of herpesvirus genomes based on bacterial genetics].Uirusu. 2004 Dec;54(2):255-64. doi: 10.2222/jsv.54.255. Uirusu. 2004. PMID: 15745165 Review. Japanese.
References
-
- Thi Nhu Thao T, Labroussaa F, Ebert N, V'Kovski P, Stalder H, Portmann J, et al. Rapid reconstruction of SARS-CoV-2 using a synthetic genomics platform [J]. Nature, 2020, 582(7813): 561–5.10.1038/s41586-020-2294-9 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
