Angiogenic and immune predictors of neoadjuvant axitinib response in renal cell carcinoma with venous tumour thrombus
- PMID: 40295487
- PMCID: PMC12037771
- DOI: 10.1038/s41467-025-58436-8
Angiogenic and immune predictors of neoadjuvant axitinib response in renal cell carcinoma with venous tumour thrombus
Abstract
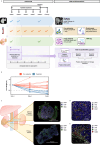
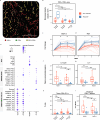
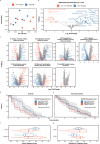
Venous tumour thrombus (VTT), where the primary tumour invades the renal vein and inferior vena cava, affects 10-15% of renal cell carcinoma (RCC) patients. Curative surgery for VTT is high-risk, but neoadjuvant therapy may improve outcomes. The NAXIVA trial demonstrated a 35% VTT response rate after 8 weeks of neoadjuvant axitinib, a VEGFR-directed therapy. However, understanding non-response is critical for better treatment. Here we show that response to axitinib in this setting is characterised by a distinct and predictable set of features. We conduct a multiparametric investigation of samples collected during NAXIVA using digital pathology, flow cytometry, plasma cytokine profiling and RNA sequencing. Responders have higher baseline microvessel density and increased induction of VEGF-A and PlGF during treatment. A multi-modal machine learning model integrating features predict response with an AUC of 0.868, improving to 0.945 when using features from week 3. Key predictive features include plasma CCL17 and IL-12. These findings may guide future treatment strategies for VTT, improving the clinical management of this challenging scenario.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare the following competing interests: H.P.: AstraZeneca (studentship). F.A.G.: research support from GE Healthcare; Grants from GSK; Consulting for AZ on behalf of the University of Cambridge. M.C.O.: 52 North Health Ltd (co-founder and employee), GE HealthCare (research funding), GSK (speaking fees). G.D.S.: Financial Interests – Evinova (consultancy, workshop on new product), British Journal of Urology International (Associate Editor) - paid role, NATCAN (Clinical Director (surgery) for the National Kidney Cancer), Audit (paid role), National Institute for Health and Care Excellence (Topic Advisor of kidney cancer guideline - paid role), AstraZeneca (Institutional Funding of the WIRE clinical trial); Non-Financial Interests - Getting It Right First Time (Principal Investigator, Chair of the kidney cancer pathway), British Association of Urological Surgeons (Member), European Association of Urology (Member); Other – MSD (Funded attendance at ESMO 2023). J.J.: Financial interests: Evinova (consultancy, workshop on new product), AstraZeneca (Institutional Funding of the WIRE clinical trial). The remaining authors declare no competing interests.
Figures

References
-
- Reese, A. C., Whitson, J. M. & Meng, M. V. Natural history of untreated renal cell carcinoma with venous tumor thrombus. Urol. Oncol. Semin. Orig. Investig.31, 1305–1309 (2013). - PubMed
-
- Martínez-Salamanca, J. I. et al. Lessons learned from the International Renal Cell Carcinoma-Venous Thrombus Consortium (IRCC-VTC). Curr. Urol. Rep.15, 404 (2014). - PubMed
-
- Woodruff, D. Y. et al. The perioperative management of an inferior vena caval tumor thrombus in patients with renal cell carcinoma. Urol. Oncol. Semin. Orig. Investig.31, 517–521 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

