Efficacy, safety and single-cell analysis of neoadjuvant immunochemotherapy in locally advanced oral squamous cell carcinoma: a phase II trial
- PMID: 40295492
- PMCID: PMC12037888
- DOI: 10.1038/s41467-025-59004-w
Efficacy, safety and single-cell analysis of neoadjuvant immunochemotherapy in locally advanced oral squamous cell carcinoma: a phase II trial
Abstract
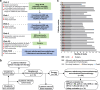
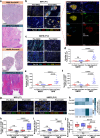
The clinical activity of neoadjuvant immunochemotherapy (NAIC) for treating locally advanced oral squamous cell carcinoma (LA-OSCC) remains uncertain. This single-arm, phase II trial (ChiCTR2200066119) tested 2 cycles of NAIC with camrelizumab plus nab-paclitaxel and cisplatin in LA-OSCC patients. For primary endpoint, the major pathological response (MPR) rate was 69.0% (95% confidence interval (CI): 49.2%-84.7%). The treatment was well-tolerated, with only 2 patients (6.45%) having grade 3 or 4 treatment-related adverse events during neoadjuvant treatment. For secondary endpoints, the pathological complete response rate was 41.4% (95%CI: 23.5%-61.1%) and the objective response rate was 82.8% (24/29, 95%CI: 64.2%-94.2%). The 18-month overall survival and disease-free survival probabilities were 96.77% (95%CI: 79.23%-99.54%) and 85.71% (95%CI: 53.95%-96.22%), respectively. Exploratory analysis showed that patients with MPR exhibited higher density of baseline CD4_Tfh_CXCL13 cells, and increased density of tertiary lymphoid structures after NAIC. Baseline CD4_Tfh_CXCL13 cells might be potential predictive biomarker of efficacy. The interaction between CXCL13 on CD4_Tfh_CXCL13 cells and CXCR5 on B cells may play a role in treatment response. These findings suggest the potential of NAIC as a promising treatment for LA-OSCC and offer preliminary insights into responsive biomarkers.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







References
-
- Chai, A. W. Y., Lim, K. P. & Cheong, S. C. Translational genomics and recent advances in oral squamous cell carcinoma. Semin. Cancer Biol.61, 71–83 (2020). - PubMed
-
- Chamoli, A. et al. Overview of oral cavity squamous cell carcinoma: Risk factors, mechanisms, and diagnostics. Oral. Oncol.121, 105451 (2021). - PubMed
-
- Chow, L. Q. M. Head and neck cancer. N. Engl. J. Med.382, 60–72 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

