Nanophotonic sensing and label-free imaging of extracellular vesicles
- PMID: 40295495
- PMCID: PMC12037801
- DOI: 10.1038/s41377-025-01866-2
Nanophotonic sensing and label-free imaging of extracellular vesicles
Abstract
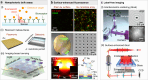
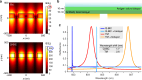
This review examines imaging-based nanophotonic biosensing and interferometric label-free imaging, with a particular focus on vesicle detection. It specifically compares dielectric and plasmonic metasurfaces for label-free protein and extracellular vesicle detection, highlighting their respective advantages and limitations. Key topics include: (i) refractometric sensing principles using resonant dielectric and plasmonic surfaces; (ii) state-of-the-art developments in both plasmonic and dielectric nanostructured resonant surfaces; (iii) a detailed comparison of resonance characteristics, including amplitude, quality factor, and evanescent field enhancement; and (iv) the relationship between sensitivity, near-field enhancement, and analyte overlap in different sensing platforms. The review provides insights into the fundamental differences between plasmonic and dielectric platforms, discussing their fabrication, integration potential, and suitability for various analyte sizes. It aims to offer a unified, application-oriented perspective on the potential of these resonant surfaces for biosensing and imaging, aiming at addressing topics of interest for both photonics experts and potential users of these technologies.
© 2025. The Author(s).
Conflict of interest statement
Conflict of interest: The authors declare no competing interests.
Figures





Similar articles
-
Dielectric metasurfaces for next-generation optical biosensing: a comparison with plasmonic sensing.Nanotechnology. 2023 Jul 19;34(40):10.1088/1361-6528/ace117. doi: 10.1088/1361-6528/ace117. Nanotechnology. 2023. PMID: 37352839 Free PMC article. Review.
-
Nanostructured Dielectric Fractals on Resonant Plasmonic Metasurfaces for Selective and Sensitive Optical Sensing of Volatile Compounds.Adv Mater. 2018 Jul;30(30):e1800931. doi: 10.1002/adma.201800931. Epub 2018 Jun 4. Adv Mater. 2018. PMID: 29862583
-
Optical Interrogation Techniques for Nanophotonic Biochemical Sensors.Sensors (Basel). 2019 Oct 3;19(19):4287. doi: 10.3390/s19194287. Sensors (Basel). 2019. PMID: 31623315 Free PMC article. Review.
-
Reviewing advances in nanophotonic biosensors.Front Chem. 2024 Sep 10;12:1449161. doi: 10.3389/fchem.2024.1449161. eCollection 2024. Front Chem. 2024. PMID: 39318420 Free PMC article. Review.
-
Patterned Plasmonic Surfaces-Theory, Fabrication, and Applications in Biosensing.J Microelectromech Syst. 2017 Aug;26(4):718-739. doi: 10.1109/JMEMS.2017.2699864. Epub 2017 May 18. J Microelectromech Syst. 2017. PMID: 29276365 Free PMC article.
Cited by
-
Small Toxic Molecule Detection and Elimination Using Molecularly Imprinted Polymers (MIPs).Biosensors (Basel). 2025 Jun 18;15(6):393. doi: 10.3390/bios15060393. Biosensors (Basel). 2025. PMID: 40558475 Free PMC article. Review.
-
Water-insensitive down-shifting nanoparticles for sensitive biosensing.Light Sci Appl. 2025 Sep 5;14(1):307. doi: 10.1038/s41377-025-01976-x. Light Sci Appl. 2025. PMID: 40913066 Free PMC article.
References
-
- Borrebaeck, C. A. K. Precision diagnostics: Moving towards protein biomarker signatures of clinical utility in cancer. Nat. Rev. Cancer17, 199–204 (2017). - PubMed
-
- Van Poppel, H. et al. Serum PSA-based early detection of prostate cancer in Europe and globally: Past, present and future. Nat. Rev. Urol.19, 562–572 (2022). - PubMed
-
- Rao, S. et al. Past, present, and future of serum tumor markers in management of ovarian cancer: A guide for the radiologist. RadioGraphics41, 1839–1856 (2021). - PubMed
Publication types
Grants and funding
- U01 CA279858/CA/NCI NIH HHS/United States
- R01 HL163513/HL/NHLBI NIH HHS/United States
- R21CA267222/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- U01CA284982/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R21 CA267222/CA/NCI NIH HHS/United States
- R01CA239078/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R01HL163513/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- U01CA279858/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R01 CA264363/CA/NCI NIH HHS/United States
- U01 CA284982/CA/NCI NIH HHS/United States
- R01 CA239078/CA/NCI NIH HHS/United States
- R01CA264363/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
LinkOut - more resources
Full Text Sources

