Automated and virus variant-programmable surrogate test qualitatively compares to the gold standard SARS-CoV-2 neutralization assay
- PMID: 40295688
- PMCID: PMC11721378
- DOI: 10.1038/s44298-024-00083-9
Automated and virus variant-programmable surrogate test qualitatively compares to the gold standard SARS-CoV-2 neutralization assay
Abstract
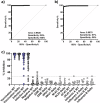
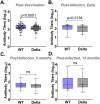
The ongoing emergence of new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants underscores the need for rapid, adaptable, high-throughput testing. However, assays for neutralizing antibodies, which are a good measure of viral protection, usually require cell culture and either infectious SARS-CoV-2 or pseudotyped viral particles. To circumvent the challenges of cell-based assays, SARS-CoV-2 surrogate virus neutralization tests (sVNTs) measure inhibition of the binding of the spike (S) protein receptor binding domain (RBD) to its receptor, human angiotensin-converting enzyme 2 (hACE2) by neutralizing antibodies. Here we tested a prototype automated microfluidic cartridge-based sVNT platform using SARS-CoV-2 wild-type (WT) and B.1.617.2 (Delta) variant RBDs. This sVNT showed a high correlation with cell-based neutralization assays for biospecimens collected post-COVID-19 vaccination and post-SARS-CoV-2 infection as well as for pre-pandemic SARS-CoV-2 negative sera. Thus, this assay, which takes less than 80 min, is a relatively simple, safe, and accurate alternative to traditional VNTs.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: C.H. and F.R. are employees of ProteinSimple, a company that designs and sells protein detection and analysis instruments. Neither C.H. or F.R. were directly involved in the laboratory or statistical analyses presented here. Potential conflicts of interest. S.D.P., T.H.B., D.R.T, and M.P.S. report that the Uniformed Services University (USU) Infectious Diseases Clinical Research Program (IDCRP), a US Department of Defense institution, and the Henry M. Jackson Foundation (HJF) were funded under a Cooperative Research and Development Agreement to conduct an unrelated phase III COVID-19 monoclonal antibody immunoprophylaxis trial sponsored by AstraZeneca. The HJF, in support of the USU IDCRP, was funded by the Department of Defense Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense to augment the conduct of an unrelated phase III vaccine trial sponsored by AstraZeneca. Both of these trials were part of the US Government COVID-19 response. Neither is related to the work presented here.
Figures



Similar articles
-
Evaluation of a biotin-based surrogate virus neutralization test for detecting postvaccination antibodies against SARS-CoV-2 variants in sera.Biochem Biophys Res Commun. 2023 Feb 26;646:8-18. doi: 10.1016/j.bbrc.2023.01.052. Epub 2023 Jan 19. Biochem Biophys Res Commun. 2023. PMID: 36696754 Free PMC article.
-
Reduced Binding between Omicron B.1.1.529 and the Human ACE2 Receptor in a Surrogate Virus Neutralization Test for SARS-CoV-2.Viruses. 2023 May 30;15(6):1280. doi: 10.3390/v15061280. Viruses. 2023. PMID: 37376580 Free PMC article.
-
Evaluation of Cell-Based and Surrogate SARS-CoV-2 Neutralization Assays.J Clin Microbiol. 2021 Sep 20;59(10):e0052721. doi: 10.1128/JCM.00527-21. Epub 2021 Jul 21. J Clin Microbiol. 2021. PMID: 34288726 Free PMC article.
-
The Biological Functions and Clinical Significance of SARS-CoV-2 Variants of Corcern.Front Med (Lausanne). 2022 May 20;9:849217. doi: 10.3389/fmed.2022.849217. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35669924 Free PMC article. Review.
-
Advances in Surrogate Neutralization Tests for High-Throughput Screening and the Point-of-Care.Anal Chem. 2025 Mar 18;97(10):5407-5423. doi: 10.1021/acs.analchem.5c00666. Epub 2025 Mar 4. Anal Chem. 2025. PMID: 40035475 Free PMC article. Review. No abstract available.
References
-
- Krammer, F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nature Medicine27, 1147–1148 (2021). - PubMed
-
- Lu, Y. et al. Advances in neutralization assays for SARS-CoV-2. Scandinav. J. Immunol.94, e13088, 10.1111/sji.13088 (2021).
-
- Pieri, M. et al. Performance evaluation of four surrogate virus neutralization tests (sVNTs) in comparison to the in vivo gold standard test. Front Biosci. 27, 74 (2022). - PubMed
Grants and funding
- HU0012020067/Defense Health Program
- HU0012020067/Defense Health Program
- HU0012020067/Defense Health Program
- HU0012020067/Defense Health Program
- HU0012020067/Defense Health Program
- HU00011920111/National Institute of Allergy and Infectious Diseases
- HU00011920111/National Institute of Allergy and Infectious Diseases
- HU00011920111/National Institute of Allergy and Infectious Diseases
- HU00011920111/National Institute of Allergy and Infectious Diseases
- HU00011920111/National Institute of Allergy and Infectious Diseases
- HU00011920111/National Institute of Allergy and Infectious Diseases
- HU00011920111/National Institute of Allergy and Infectious Diseases
LinkOut - more resources
Full Text Sources
Miscellaneous

