Somatic mutations and outcomes in chronic myeloid leukemia adolescent and young adults compared to children, adults, and BCR::ABL1-positive acute lymphoblastic leukemia
- PMID: 40295826
- PMCID: PMC12208900
- DOI: 10.1038/s41375-025-02609-3
Somatic mutations and outcomes in chronic myeloid leukemia adolescent and young adults compared to children, adults, and BCR::ABL1-positive acute lymphoblastic leukemia
Abstract
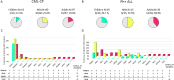
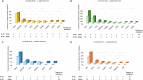
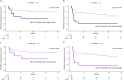
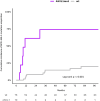
Adolescent and young adults (AYAs) with chronic myeloid leukemia in chronic phase (CML-CP) reportedly respond worse to tyrosine kinase inhibitors (TKIs) than adults, potentially due to additional genetic abnormalities, including mutations in cancer-related genes (CRGs). This real-life study compared mutation profiles and their impact on outcomes in 80 AYA, 97 adult, and 16 pediatric CML-CP patients, alongside 81 BCR::ABL1-positive acute lymphoblastic leukemia (Ph+ ALL) patients. CRG mutations were more frequent in AYAs (25.0%) than in adults (19.6%) or children (12.5%). AYAs with Ph+ ALL exhibited higher mutational frequencies (53.3%) compared to children (26.7%) and adults (38.9%). At diagnosis, mutations in ASXL1, DNMT3A, and TET2 dominated in CML-CP and RUNX1, IKZF1, and BCR::ABL1 in Ph+ ALL. ASXL1 mutations correlated with reduced progression-free survival (PFS) in AYAs and adults. Unlike adults, AYAs showed no increase in BCR::ABL1 kinase domain mutations during TKI therapy. Nilotinib improved PFS in AYAs with ASXL1 mutations, highlighting the efficacy of higher-generation TKIs. ASXL1 mutations also impaired erythropoiesis, warranting further validation. Despite a higher mutational burden, AYAs did not exhibit worse prognoses than adults. Lower mutation rates at follow-up suggest potential impact of nilotinib. Mutation profiling and optimized TKI use are crucial to mitigate progression risks in CRG-mutated patients.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: KMP—Novartis - advisory board and research support. The remaining authors declare no competing financial interests. Ethics approval: The project was approved by the local Ethics Review Committee (EK 1/AZV ČR/06/2020). Informed consent: All patients, as well as the parents of paediatric patients, provided written informed consent for the use of their samples and clinical data in the research project, in accordance with the Declaration of Helsinki and institutional guidelines.
Figures
References
-
- Saglio G, Kim D-W, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–9. - PubMed
-
- National Cancer Institute SEER USA. SEER USA. https://seer.cancer.gov/statfacts/html/cmyl.html. Accessed 18 Dec 2024.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

