Body weight-supported treadmill training reduces glial scar overgrowth in SCI rats by decreasing the reactivity of astrocytes during the subacute phase
- PMID: 40295901
- PMCID: PMC12039159
- DOI: 10.1186/s12868-025-00947-7
Body weight-supported treadmill training reduces glial scar overgrowth in SCI rats by decreasing the reactivity of astrocytes during the subacute phase
Abstract
Background: Spinal cord injury is followed by glial scar formation, which was long seen mainly as a physical barrier preventing axonal regeneration. Glial scar astrocytes lead to glial scar formation and produce inhibitory factors to prevent axons from growing through the scar, while inhibiting the conversion of reactive astrocytes into glial scar-forming astrocytes may represent an ideal treatment for CNS injury. Exercise is a non-invasive and effective therapeutic intervention for clinical rehabilitation of spinal cord injury. However, its precise therapeutic mechanisms still need to be continuously explored.
Methods: 30 rats were randomly assigned to three groups (Sham, SCI, SCI + BWSTT; n = 10 rats per group). In this study, we employed the BBB scales and gait analysis system to examine the behavioral functions of the rats in each group. Furthermore, we utilized immunoblotting of spinal cord tissue at the injury site, in addition to histological staining and immunofluorescence staining, to explore glial scar aggregation and axonal regeneration in each group of rats.
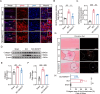
Results: Our results revealed that hindlimb motor function was significantly improved in SCI rats after a sustained subacute period of BWSTT, accompanied by the promotion of histological repair and nerve regeneration. Subsequent immunofluorescence staining and immunoblotting showed diminished astrocyte reactivity in the region surrounding the spinal cord injury as well as reduced expression and distribution of collagen fibers near the lesion after BWSTT. Additionally, a significant decrease in the expression of MMP-2/9, which is closely related to astrocyte migration, was observed in the vicinity of spinal cord tissue lesions.
Conclusion: Our study demonstrates that a sustained BWSTT intervention during the subacute phase of spinal cord injury can effectively reduce astrocyte reactivity and glial scarring overgrowth, thereby facilitating functional recovery after SCI.
Keywords: Astrocyte reactivity; Body weight-supported treadmill training; Glial scar; Matrix metalloproteinase-2/9; Spinal cord injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experiments involving animals were approved by the Ethics Committee for Animal Experiments of Nanjing Medical University (license No. IACUC-2206045 and No. IACUC-2403019), Jiangsu Province, China. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

