Unveiling the heterogeneity and immunotherapy potency of tumor-associated neutrophils in the tumor microenvironment of gastric cancer
- PMID: 40295944
- PMCID: PMC12036284
- DOI: 10.1186/s12876-025-03920-0
Unveiling the heterogeneity and immunotherapy potency of tumor-associated neutrophils in the tumor microenvironment of gastric cancer
Abstract
Background: The differentiation characteristics of neutrophils within the gastric cancer (GC) tumor microenvironment (TME) and their interactions with malignant gastric epithelial cells require further investigation. Furthermore, the therapeutic potential of tumor-associated neutrophils (TANs) in immunotherapy remains inadequately explored.
Methods: We integrated two single-cell transcriptome datasets comprising 12 samples, including gastric primary tumors, non-tumor tissues, and metastatic tumors, to profile the epithelial cells and TANs atlas within the TME and examine their interaction modules. In addition, these data were integrated with the bulk transcriptomic including the Cancer Genome Atlas - Stomach Adenocarcinoma (TCGA-STAD) and Asian Cancer Research Group (ACRG) datasets to analyze the expression levels of neutrophil-associated genes across the tumor-associated neutrophil subsets.
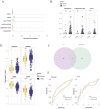
Results: We analyzed 3,118 gastric epithelial cells and 2,365 TANs from all samples. Epithelial cells were classified into ten subclusters, while TANs were grouped into five subclusters. In gastric primary tumors, epithelial cell subtypes included primarily MUC16 + and stem-like populations. In metastatic tumors, the epithelial cell subset with high CXCL5 expression was a characteristic subtype. TANs mainly interacted with epithelial cells via the LGALS9-CD45 and CD46-JAG1 pathways. And RGS2 was highly expressed in N4, a tumor-associated neutrophils subcluster characterized by high MMP9 expression, highlighting its potential as an immunotherapy target.
Conclusion: TANs exhibit robust interactions with gastric malignant epithelial cell subsets. Furthermore, RGS2, which is highly expressed in N4, could serve as a promising target for immunotherapy.
Keywords: Gastric cancer; Gastric metastatic tumor; ScRNAseq; Tumor microenvironment; Tumor-associated neutrophils.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was based entirely on publicly available datasets. No experiments involving human participants or animals were conducted by the authors. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- llemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–75. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- National Natural Science Foundation of China/National Natural Science Foundation of China
- No.2023GXNSFBA026164/National Natural Science Foundation of Guangxi
- No.AB24010149/Guangxi Key Research and Development Project
- No.AD19245196/Guangxi Clinical Research Center for Enhanced Recovery after Surgery, Guangxi Science and Technology Base and Talent Project
- No.YYZS2020003/Guangxi key Laboratory of Enhanced Recovery after Surgery for Gastrointestinal Cancer
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

