Evaluation of timed dexamethasone eye drops to prevent proliferative retinopathy of prematurity: a study protocol for a randomized intervention, multi-centre, double-blinded trial (DROPROP)
- PMID: 40295974
- PMCID: PMC12036246
- DOI: 10.1186/s12887-025-05673-x
Evaluation of timed dexamethasone eye drops to prevent proliferative retinopathy of prematurity: a study protocol for a randomized intervention, multi-centre, double-blinded trial (DROPROP)
Abstract
Background: As the survival rate of preterm infants continues to rise worldwide, more infants are at risk of developing sight-threatening retinopathy of prematurity (ROP). Destructive retinal laser treatment and intravitreal injections of anti-vascular endothelial growth factor (VEGF), factor, which have potential systemic side effects, are necessary to prevent blindness in severe cases of ROP. Off-label use in clinical settings suggests that dexamethasone eye drops, 1 mg/ml, may prevent the progression of ROP to severe disease (Type 1 ROP) requiring treatment. Our current study aims to assess the efficacy and safety of timely administered dexamethasone eye drops to reduce the need for laser or anti-VEGF ROP treatment in preterm infants.
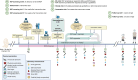
Methods: In a randomized prospective interventional, multi-centre, double-blinded trial, we plan to include 100 infants with severe ROP born before gestational age 30 weeks in Sweden. Infants will be randomized to intervention with dexamethasone eye drops (1 mg/ml) (n = 50) or placebo, saline (n = 50) until either ROP is resolved or severe ROP (Type 1 ROP) development occurs, fulfilling ROP treatment criteria. Eye drops will be administered one drop per day or every other day, depending on the severity of ROP, with a maximum duration of 12 weeks. The primary objective is to evaluate whether dexamethasone intervention reduces the proportion of infants developing Type 1 ROP compared to infants receiving a placebo. Adverse events and potential side effects will be recorded, such as high intraocular pressure and growth restriction. Levels of cortisol in saliva and glucose in urine will be measured repeatedly. Secondary outcomes will include the timing of ROP progression, the recurrence rate after ROP treatment and retinal morphology. An ophthalmological follow-up will be initiated at 2 and 5.5 years of age, evaluating visual acuity, refractive errors, strabismus, retinal morphology and ophthalmological complications. All outcomes in the study will be compared between the infants receiving dexamethasone intervention and those receiving placebo.
Discussion: Timely administration of dexamethasone eye drops may prevent severe ROP from progressing to Type 1 ROP, which requires treatment. This study aims to assess the efficacy and safety of dexamethasone intervention to support its clinical use and national guidelines.
Trial registration: EudraCT, 2020-004933-19, registered in January 2021 and CTIS, 2023-505318-97-00, registered in August 2023.
Clinical trial number: Not applicable.
Keywords: Dexamethasone; Eye drops; Preterm infants; Retinopathy of prematurity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This manuscript represents the content of protocol version 4, October 2022. Ethical approval of this study was obtained by the Swedish Ethical Review Authority, Ethical initial application Dnr 2020–06028 approved 2021/02/23, amendment Dnr 2022–02641 - 02 approved 2022/06/06 and amendment Dnr 2023–03225 - 02 approved 2023/06/29. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- ALFGBG-812951, ALFGBG-971188, ALF 2022-YF0008/the Swedish state under the agreement between the Swedish government and the county councils - the ALF-agreement
- ALFGBG-812951, ALFGBG-971188, ALF 2022-YF0008/the Swedish state under the agreement between the Swedish government and the county councils - the ALF-agreement
- ALFGBG-812951, ALFGBG-971188, ALF 2022-YF0008/the Swedish state under the agreement between the Swedish government and the county councils - the ALF-agreement
- KAW 2018.0310/The Wallenberg Clinical Scholars
- KAW 2020.0239/the SciLifeLab & Wallenberg Data Driven Life Science Program
LinkOut - more resources
Full Text Sources
Medical
Research Materials