Exchange of the L-cysteine exporter after in-vivo metabolic control analysis improved the L-cysteine production process with engineered Escherichia coli
- PMID: 40296075
- PMCID: PMC12038998
- DOI: 10.1186/s12934-025-02715-y
Exchange of the L-cysteine exporter after in-vivo metabolic control analysis improved the L-cysteine production process with engineered Escherichia coli
Abstract
Background: L-Cysteine is a proteinogenic amino acid of high pharmaceutical and industrial interest. However, the fermentation process for L-cysteine production is faced with multiple obstacles, like the toxicity of L-cysteine for the cells, the low carbon yield of the product, and the low selectivity of the L-cysteine exporter. In previous work, in-vivo metabolic control analysis (MCA) applied to an L-cysteine fed-batch production process with E. coli, followed by the targeted metabolic engineering to reduce an intracellular O-acetylserine (OAS) deficiency, resulted in a significant improvement of the L-cysteine production process with the new producer strain.
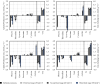
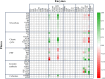
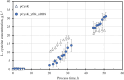
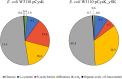
Results: In this work, in-vivo MCA was applied to the L-cysteine fed-batch production process with the new producer strain (E. coli W3110 pCysK). The MCA indicated that a simultaneous increase in the exporter's expression and selectivity is required to increase the L-cysteine production further. The exchange of the L-cysteine exporter YdeD present in the plasmid pCysK for the potentially more selective exporter YfiK led to an increase of the maximal L-cysteine concentration by the end of the fed-batch process of 37% to a final concentration of 33.8 g L-1. The L-cysteine production could also be extended for at least 20 h due to conserved cellular activity as a result of the reduction of carbon loss as OAS.
Conclusions: It could be shown that the in-vivo MCA methodology can be utilised iteratively with cells from the production process to pinpoint targets for further strain optimisation towards a significant increase in the L-cysteine production with E. coli. The use of this technology in combination with process engineering to adapt the fed-batch process to the modified strain may achieve a further improvement of the process performance.
Keywords: E. coli; l-cysteine; l-cysteine exporter; Dual substrate feeding; Fed-batch process; Metabolic control analysis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: Not applicable. Consent to participate: Not applicable. Consent for publication: Not applicable.
Figures



Similar articles
-
Metabolic control analysis enabled the improvement of the L-cysteine production process with Escherichia coli.Appl Microbiol Biotechnol. 2024 Dec;108(1):108. doi: 10.1007/s00253-023-12928-z. Epub 2024 Jan 11. Appl Microbiol Biotechnol. 2024. PMID: 38212968 Free PMC article.
-
L-Cysteine Production in Escherichia coli Based on Rational Metabolic Engineering and Modular Strategy.Biotechnol J. 2018 May;13(5):e1700695. doi: 10.1002/biot.201700695. Epub 2018 Feb 23. Biotechnol J. 2018. PMID: 29405609
-
YfiK from Escherichia coli promotes export of O-acetylserine and cysteine.J Bacteriol. 2003 Feb;185(4):1161-6. doi: 10.1128/JB.185.4.1161-1166.2003. J Bacteriol. 2003. PMID: 12562784 Free PMC article.
-
Metabolic control analysis enables rational improvement of E. coli L-tryptophan producers but methylglyoxal formation limits glycerol-based production.Microb Cell Fact. 2022 Oct 4;21(1):201. doi: 10.1186/s12934-022-01930-1. Microb Cell Fact. 2022. PMID: 36195869 Free PMC article.
-
Metabolic engineering of Escherichia coli for efficient production of l-arginine.Adv Appl Microbiol. 2023;122:127-150. doi: 10.1016/bs.aambs.2022.11.002. Epub 2022 Dec 20. Adv Appl Microbiol. 2023. PMID: 37085192 Review.
References
-
- Hashim Y, Ismail N, Jamal P, Othman R, Salleh H. Production of cysteine: approaches, challenges and potential solution. Int J Biotech Well Indus. 2014;3(3):95–101.
-
- Powell BC, Walker SK, Bawden CS, Sivaprasad AV, Rogers GE. Transgenic sheep and wool growth: possibilities and current status. Reprod Fertil Dev. 1994;6(5):615–23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

