Can the CANKADO online application improve quality of life monitoring via the endometriosis health profile-30 in endometriosis patients: A randomized cohort study on acceptance, usability, and correlations with demographics and media usage
- PMID: 40296083
- PMCID: PMC12036296
- DOI: 10.1186/s12905-025-03736-w
Can the CANKADO online application improve quality of life monitoring via the endometriosis health profile-30 in endometriosis patients: A randomized cohort study on acceptance, usability, and correlations with demographics and media usage
Abstract
Background: The global increase in interest in endometriosis highlights the importance of further investigations concerning this so-called benign gynecological disease. Owing to their severe presentation of symptoms, patients suffer from an enormous impact on their health-related quality of life (HRQoL). While the paper-based assessment of quality of life via, e.g., the "Endometriosis Health Profile-30 questionnaire (EHP-30)" seems to be largely accepted and implemented, the electronic measurement of this patient-reported outcome (ePRO) is still rarely applied. This study aimed to analyze the acceptance and usability of electronic assessments of HRQoL in endometriosis patients via the online platform CANKADO.
Methods: The study was conducted at the Department of Gynecology and Obstetrics of LMU Munich between January 2022 and February 2023. Sixty conservatively treated patients with endometriosis were recruited for the randomized cohort study, followed by randomization due to their planned interrogation modality (n paper-based = 23, n online-based = 17). Afterwards, a HRQoL assessment via the EHP-30 questionnaire was performed. An evaluation of the interrogation modalities was performed at 0, 6 and 12 months. The metric or categorical variables were compared via Fisher's exact test or the Mann‒Whitney U test. Correlation analysis was performed by calculating the Kendall Tau coefficient or Eta coefficient.
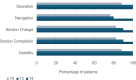
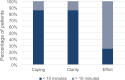
Results: Forty patients completed evaluation forms at T0 (0 months), with n = 23 evaluating the paper-based interrogation modality and n = 17 evaluating the online version. At all the time of assessment, more than 80% of the patients showed a positive response to routinely performed ePRO measurements in the clinical context, expecting simplified communication, faster diagnosis, and therapeutic improvement. The online modality was rated more suitably (T0: 72.7% vs. 76.5%; T3: 60.0% vs. 90.0%), less complex (T0: 59.1% vs. 76.5%; T3: 80.0% vs. 70.0%), and less laborious (T0: 72.7% vs. 70.6%; T3: 80% each). Completion time over ten minutes was significantly correlated with low coping ability (r = 0.530; p = 0.029), lower clarity (r = 0.530; p = 0.029) and greater effort (r = 0.593; p = 0.012).
Conclusions: The findings indicate high acceptance and usability of regularly performed ePRO assessments in patients with endometriosis via the online tool CANKADO.
Keywords: CANKADO; EHP-30; Electronic patient-reported outcome measurement; Endometriosis; Health-related quality of life.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Informed consent was obtained from all patients, and all the patients participated voluntarily. Anonymity was assured. This study (project number: 21–0354) was approved on May 17, 2021, by the Research Ethics Committee of LMU Munich (Ethikkommission bei der LMU München), and all assessments were in accordance with the Helsinki Declaration. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Keilmann L, Beyer S, Meister S, Jegen M, Buschmann C, Schröder L, et al. Trends among patients with endometriosis over a 7-year period and the impact of the COVID-19 pandemic: experience from an academic high-level endometriosis centre in Germany. Arch Gynecol Obstet. 2023;307:129–37. 10.1007/s00404-022-06730-x - DOI - PMC - PubMed
-
- Olliges E, Bobinger A, Weber A, Hoffmann V, Schmitz T, Popovici RM, Meissner K. The physical, psychological, and social day-to-day experience of women living with endometriosis compared to healthy age-matched controls-a mixed-methods study. Front Glob Womens Health. 2021;2:767114. 10.3389/fgwh.2021.767114 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical