From infection to infertility: a review of the role of human papillomavirus-induced oxidative stress on reproductive health and infertility
- PMID: 40296084
- PMCID: PMC12036311
- DOI: 10.1186/s40001-025-02605-4
From infection to infertility: a review of the role of human papillomavirus-induced oxidative stress on reproductive health and infertility
Abstract
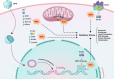
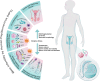
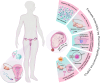
Infertility has emerged as a significant global health concern, affecting nearby 8-12% of couples in reproductive age worldwide. Increasing evidence suggests a potential link between human papillomavirus (HPV) and infertility in both men and women. Some research indicate that HPV can infect various components of semen, potentially affecting sperm quality by decreasing motility, viability, and increasing DNA fragmentation, all of which may contribute to male infertility. The virus can attach to the equatorial region of the sperm head, enabling infected sperm to transmit the virus to the oocyte or placenta. Consequently, HPV potentially induces apoptosis in trophoblastic cells and disrupts their adhesion to endometrial cells, which raises the risk of miscarriage. HPV may also affect ovarian reserve by causing chronic inflammation, which can impair granulosa cell function and lower serum anti-Müllerian hormone (AMH) levels. Besides, HPV-related immune responses also contribute to infertility by producing anti-sperm antibodies (ASAs), which cause sperm clumping, reduce motility through cervical mucus, activate the complement system that damages sperm in the female reproductive tract and interfere with sperm-egg interactions. Moreover, HPV infection has been linked to reduced success rates in assisted reproductive technologies (ART), potentially disrupting critical processes such as the acrosome reaction, sperm-oocyte interaction, and fusion. One potential mechanism through which HPV contributes to infertility is oxidative stress (OS). Triggered OS can negatively impact sperm quality and cause damage to the female reproductive system, ultimately contributing to infertility. Despite these associations, the precise mechanisms and the strength of the relationship remain uncertain. Thus, this review seeks to investigate the potential impact of HPV on infertility, particularly its effects on the reproductive system through OS. A clearer understanding of these processes could inform future health strategies for addressing HPV-related infertility.
Keywords: Human papillomavirus; Infertility; Inflammation; Oxidative stress; Reproductive health; Reproductive tract infections.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable Consent for publication: Not applicable Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
The role of human papillomavirus on sperm function.Curr Opin Obstet Gynecol. 2011 Aug;23(4):232-7. doi: 10.1097/GCO.0b013e328348a3a4. Curr Opin Obstet Gynecol. 2011. PMID: 21666465 Review.
-
Human Papillomavirus and Male Infertility: What Do We Know?Int J Mol Sci. 2023 Dec 16;24(24):17562. doi: 10.3390/ijms242417562. Int J Mol Sci. 2023. PMID: 38139389 Free PMC article. Review.
-
Exploring the potential impact of human papillomavirus on infertility and assisted reproductive technology outcomes.Reprod Biol. 2023 Jun;23(2):100753. doi: 10.1016/j.repbio.2023.100753. Epub 2023 Mar 6. Reprod Biol. 2023. PMID: 36889139 Review.
-
Seminal human papillomavirus infection and reproduction: a systematic review and meta-analysis.Andrology. 2021 Mar;9(2):478-502. doi: 10.1111/andr.12948. Epub 2020 Dec 11. Andrology. 2021. PMID: 33220146
-
The impact of human papilloma virus on human reproductive health and the effect on male infertility: An updated review.J Med Virol. 2023 Apr;95(4):e28697. doi: 10.1002/jmv.28697. J Med Virol. 2023. PMID: 36951428 Review.
References
-
- Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10. - PubMed
-
- Wasilewski T, Łukaszewicz-Zając M, Wasilewska J, Mroczko B. Biochemistry of infertility. Clin Chim Acta. 2020;508:185–90. - PubMed
-
- Walker MH, Tobler KJ. Female infertility. Treasure Island (FL): StatPearls Publishing; 2020. - PubMed
-
- Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab. 2011;25(2):271–85. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

