Safety and effectiveness of switch to bictegravir/emtricitabine/tenofovir alafenamide following dual regimen therapy in people with HIV: Insights from the Icona cohort
- PMID: 40296167
- PMCID: PMC12127237
- DOI: 10.1111/hiv.70037
Safety and effectiveness of switch to bictegravir/emtricitabine/tenofovir alafenamide following dual regimen therapy in people with HIV: Insights from the Icona cohort
Abstract
Objectives: Most treatment switches are for simplification from three-drug (3DR) to dual regimens (2DR). However, a proportion of people with HIV may switch back to 3DR, like bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) after 2DR.
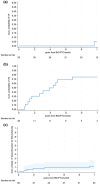
Methods: We included people with HIV enroled in the Icona cohort who switched to B/F/TAF after 2DR INSTI-based (3TC/DTG, RPV/DTG, RPV/CAB, or DOR + DTG). Virological rebound (VR), virological suppression (VS), and treatment discontinuation (TD) due to toxicity or failure were evaluated using Kaplan-Meier curves. Random intercept and slopes before and after the switch were used to evaluate the trajectories of triglycerides, cholesterol, CD4, and CD4/CD8. Viro-immunological analyses were stratified according to HIV-RNA at switch.
Results: Among the 3662 people with HIV who started a 2DR INSTI-based regimen, 71 (1.9%) switched to B/F/TAF; 60 had been followed up after the switch, for a median of 10.9 months (interquartile range: 3.6-24.7). Forty people with HIV switched with HIV-RNA <50 copies/mL (uVL), 20 with HIV-RNA ≥50 copies/mL (dVL). Among the uVL group, one participant experienced VR (HIV-RNA: 99, 71 followed by 29 copies/mL). Among the dVL group, the 1-year cumulative probability of undetectable VL was 75% (95% confidence interval [CI] 57.6-95.1). Fourteen people with HIV interrupted B/F/TAF for simplification (50.0%), toxicity (28.6%), VR (14.2%), and patient's choice (7.1); the 1-year cumulative probability of TD for toxicity/failure was 10.7% (95% CI 14.5-24.5). We observed an increase in the CD4/CD8 ratio (+0.02 CD4/CD8/month, p = 0.026) only in the dVL group.
Conclusions: Switching from 2DR-INSTI to B/F/TAF is infrequent; this switch results in a low rate of toxicity and failure, along with a favourable immunovirological and lipid profile. CD4/CD8 gain is observed in those switching with detectable HIV-RNA.
Keywords: ART switch; bictegravir; dual therapy; viral load.
© 2025 The Author(s). HIV Medicine published by John Wiley & Sons Ltd on behalf of British HIV Association.
Conflict of interest statement
Alessandro Tavelli, Alessandro Cozzi‐Lepri, Alice Ianniello, Antonella d'Arminio Monforte, Nicoletta Bobbio, Giacomo Ponta declare no conflicts of interest. Andrea De Vito received fees for attending advisory boards from ViiV. Andrea Giacomelli received fees for attending advisory boards from ViiV. Speakers' honoraria from Gilead, Janssen, MSD, and ViiV. Roberto Rossotti received grants, fees for speaker's bureau, and advisory board activities from ViiV Healthcare, MSD, Gilead Sciences, Johnson & Johnson, and SD Biosensor. Antonella Cingolani received funding for scientific advisory boards, travel, or speaker honoraria from Gilead Sciences, ViiV Healthcare, Janssen‐Cilag, and MSD. Giordano Madeddu received speaker's honoraria and fees for attending advisory boards from Viiv, Gilead, MSD, Janssen, and Tera Technologies. Andrea Antinori served as a paid consultant to Astra Zeneca, Bavarian Nordic, Gilead Sciences, GSK, Janssen‐Cilag, MSD, Moderna, Pfizer, and ViiV Healthcare and received institutional research grants from Astra Zeneca, Gilead Sciences, and ViiV Healthcare.
Figures
References
-
- EACS European AIDS Clinical Society . EACS Guidelines 12.0. 2023.
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. 2024. Accessed December 10, 2024. https://clinicalinfo.hiv.gov/
-
- Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral‐naive adults with HIV‐1 infection (GEMINI‐1 and GEMINI‐2): week 48 results from two multicentre, double‐blind, randomized, non‐inferiority, phase 3 trials. Lancet. 2019;393(10167):143‐155. - PubMed
-
- Van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed‐dose 2‐drug regimen vs continuing a tenofovir alafenamide‐based 3‐ or 4‐drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, non‐inferiority TANGO study. Clin Infect Dis. 2020;71(8):1920‐1929. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

