Inter-Ethnic Differences in the Efficacy and Safety of Tyrosine Kinase Inhibitors Used in Oncology: Insights From Phase 3 Clinical Trials
- PMID: 40296413
- PMCID: PMC12037692
- DOI: 10.1111/cts.70224
Inter-Ethnic Differences in the Efficacy and Safety of Tyrosine Kinase Inhibitors Used in Oncology: Insights From Phase 3 Clinical Trials
Abstract
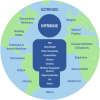
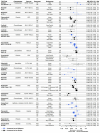
Differences in the efficacy and safety of tyrosine kinase inhibitors (TKIs) have been observed across ethnic/ancestry subpopulations (previously reviewed to 2017). With an expanding number of TKIs approved since that time, an updated review of TKI response across ethnic/ancestry subpopulations in Phase 3 TKI clinical trials was conducted. A total of 73 population subgroup analyses (defined by participant race, ethnicity, ancestry or geographic region) of progression-free survival (PFS) and/or overall survival (OS) were identified by a literature search. Twelve (16%) of the analyses investigating the efficacy of afatinib, brigatinib, dacomitinib, gilteritinib, lorlatinib, neratinib, osimertinib, or pazopanib were assessed to report population differences in PFS and/or OS. For 28 (38%) of the analyses that showed suggestions of a potential efficacy difference across subpopulations, limitations in the data available precluded further assessment. There were 17 (23%) analyses assessed to report comparable efficacy outcomes across diverse subpopulations. The majority of clinical trials noted no clinically remarkable differences in safety between subpopulations; however, for brigatinib, crizotinib, pazopanib, and sunitinib, distinct patterns of adverse events were reported in the Asian and non-Asian subgroups. The underrepresentation of specific subpopulations, the grouping together of results of diverse subpopulations, as well as inconsistencies in the definition and reporting of participant ethnicity/ancestry are barriers to the meaningful exploration of inter-ethnic differences in TKI response. Therefore, further insight into the associations between ethnicity/ancestry and TKI response will require an increase in the diversity of clinical trial participants and appropriate analysis and reporting of subpopulation results.
Keywords: diversity; efficacy; ethnicity; geographic ancestry; inter‐ethnic differences; safety; tyrosine kinase inhibitors.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Presentation: Nicki M. Kyriacou, Annette S. Gross, Andrew J. McLachlan (Poster 512): Ethnic differences in the safety and efficacy of tyrosine kinase inhibitors. Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists 2023 Conference, Sydney, Australia, 20th–23rd November 2023.
The authors declare no conflicts of interest.
Figures

References
-
- Touma J. A., McLachlan A. J., and Gross A. S., “The Role of Ethnicity in Personalized Dosing of Small Molecule Tyrosine Kinase Inhibitors Used in Oncology,” Translational Cancer Research 6, no. S10 (2017): S1558–S1591, 10.21037/tcr.2017.09.09. - DOI
-
- Rowland A., Van Dyk M., Mangoni A. A., et al., “Kinase Inhibitor Pharmacokinetics: Comprehensive Summary and Roadmap for Addressing Inter‐Individual Variability in Exposure,” Expert Opinion on Drug Metabolism & Toxicology 13 (2017): 31–49. - PubMed
-
- Levitzki A. and Gazit A., “Tyrosine Kinase Inhibition: An Approach to Drug Development,” Science 267 (1995): 1782–1788. - PubMed
-
- Huang S. M. and Temple R., “Is This the Drug or Dose for You?: Impact and Consideration of Ethnic Factors in Global Drug Development, Regulatory Review, and Clinical Practice,” Clinical Pharmacology and Therapeutics 84 (2008): 287–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

