Patient-derived tumor organoids highlight the potential of precision medicine in managing pancreatic ductal adenocarcinoma
- PMID: 40296444
- PMCID: PMC12178096
- DOI: 10.1002/ijc.35443
Patient-derived tumor organoids highlight the potential of precision medicine in managing pancreatic ductal adenocarcinoma
Abstract
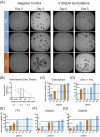
Pancreatic ductal adenocarcinoma (PDAC) ranks among the most lethal cancers, with only 20% of patients qualifying for curative treatment at diagnosis. Three-dimensional tumor organoids capturing patient-specific features of PDAC serve as a valuable disease model. We employed this technology to assess drug sensitivities of patient-derived tumor organoids to clinically relevant drugs and combinations, evaluated culture success rates, and correlated in vitro data with clinicopathological and follow-up information. Tumor organoid cultures were established from PDAC patients undergoing surgical resection (or liver biopsy) and follow-up at a single medical center. Patient-derived cultures displaying sustained growth were analyzed regarding their molecular subtype and utilized for functional drug sensitivity testing (f-DST). Correlative analyses of our PDAC patient cohort (n = 67; n = 42 patients with curative tumor resection and n = 25 palliative patients) revealed a link between tumor organoid growth and reduced patient survival. Furthermore, drug sensitivity profiles (obtained of 10 patient-derived cultures) revealed notable inter-individual differences and mirrored clinical responses to administered drug therapies. f-DST was applicable across tumor organoid cultures of both classical and basal subtype, according to the Purity Independent Subtyping of Tumors (PurIST) classifier. This pilot study confirms the feasibility of deriving and maintaining tumor organoid cultures from heterogeneous samples. Cultures displaying sustained proliferation correlated positively with advanced-stage tumors (Tumour, Node, Metastasis (UICC) stages III and IV). Individual patient case analyses integrating in vitro drug sensitivity profiles with clinical follow-up data suggest that f-DST using tumor organoids could guide future therapeutic strategies. In summary, tumor organoids offer insights into patient-specific responses to treatment, highlighting the potential of precision medicine in managing this challenging cancer.
Keywords: drug testing; pancreatic cancer; precision medicine; tumor organoids.
© 2025 The Author(s). International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
Employment or Leadership: Chief Technology Officer of 2cureX (Jacob Thastrup). Stock Ownership: Ownership of 2cureX stocks (Jacob Thastrup, Jürgen Kupper). All other authors declare no conflicts of interest.
Figures


References
-
- Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008;9(2):99‐132. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

