Advancements in systemic therapy for muscle-invasive bladder cancer: A systematic review from the beginning to the latest updates
- PMID: 40296876
- PMCID: PMC12035237
- DOI: 10.1177/23523735251335122
Advancements in systemic therapy for muscle-invasive bladder cancer: A systematic review from the beginning to the latest updates
Abstract
Context: Several phase III randomized controlled trials (RCTs) have shown the importance of perioperative systemic therapy, especially for the efficacy of immune checkpoint inhibitors (ICIs) in both neoadjuvant and adjuvant settings for muscle-invasive bladder cancer (MIBC).
Objective: To synthesize the growing evidence on the efficacy and safety of systemic therapies for MIBC utilizing the data from RCTs.
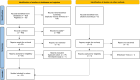
Evidence acquisition: Three databases and ClinicalTrials.gov were searched in October 2024 for eligible RCTs evaluating oncologic outcomes in MIBC patients treated with systemic therapy. We evaluated pathological complete response (pCR), disease-free survival (DFS), progression-free survival (PFS), event-free survival (EFS), overall survival (OS), and adverse events (AEs).
Evidence synthesis: Thirty-three RCTs (including 14 ongoing trials) were included in this systematic review. Neoadjuvant chemotherapy improved OS compared to radical cystectomy alone. Particularly, the VESPER trial demonstrated that dd-MVAC provided oncological benefits over GC alone in terms of pCR rates, OS (HR: 0.71), and PFS (HR: 0.70). Recently, the NIAGARA trial showed that perioperative durvalumab plus GC outperformed GC alone in terms of pCR rates, OS (HR: 0.75), and EFS (HR: 0.68). Despite the lack of data on overall AE rates in the VESPER trial, differential safety profiles in hematologic toxicity were reported between dd-MVAC and durvalumab plus GC regimens. In the adjuvant setting, no study provided the OS benefit from adjuvant chemotherapy. However, only adjuvant nivolumab had significant DFS and OS benefits compared to placebo.
Conclusions: Neoadjuvant chemotherapy remains the current standard of care for MIBC. Durvalumab shed light on the promising impact of ICIs added to neoadjuvant chemotherapy. Nivolumab is the only ICI recommended as adjuvant therapy in patients who harbored adverse pathologic outcomes. Ongoing trials will provide further information on the impact of combination therapy, including chemotherapy, ICIs, and enfortumab vedotin, in both neoadjuvant and adjuvant settings.
Keywords: adjuvant; chemotherapy; immune checkpoint inhibitors; muscle-invasive; neoadjuvant; urothelial carcinoma.
© The Author(s) 2025.
Figures
References
-
- Hautmann RE, de Petriconi RC, Pfeiffer C, et al. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol 2012; 61: 1039–1047. - PubMed
-
- Leow JJ, Chong YL, Chang SL, et al. Neoadjuvant and adjuvant chemotherapy for upper tract urothelial carcinoma: a 2020 systematic review and meta-analysis, and future perspectives on systemic therapy. Eur Urol 2021; 79: 635–654. - PubMed
-
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001; 19: 666–675. - PubMed
-
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003; 349: 859–866. - PubMed
-
- Lehmann J, Franzaring L, Thüroff J, et al. Complete long-term survival data from a trial of adjuvant chemotherapy vs control after radical cystectomy for locally advanced bladder cancer. BJU Int 2006; 97: 42–47. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous