Mycobacterial antigen Ag85B restrains Hodgkin lymphoma tumor growth by inhibiting autophagy
- PMID: 40296899
- PMCID: PMC12034006
- DOI: 10.32604/or.2025.057842
Mycobacterial antigen Ag85B restrains Hodgkin lymphoma tumor growth by inhibiting autophagy
Abstract
Background: The growth of the B-cell lymphoma subtype, Hodgkin lymphoma (HL), is associated with increased autophagy. A mycobacterial antigen, Ag85, has been reported to inhibit cell autophagy under a variety of conditions. Whether Ag85 could inhibit autophagy in HL is unknown.
Methods: Lymph node samples from patients with HL and healthy controls were collected to assess proliferation and autophagy. The human HL cell line, L-428, was cultured and subjected to Ag85B treatment. Autophagy in L-428 cells was evaluated through western blotting analysis, immunohistochemistry, and transmission electron microscopy. Apoptosis in these cells was measured using flow cytometry and western blotting. The associated signaling pathways were also analyzed utilizing western blotting. The in vivo impact of Ag85B was studied using BALB/c Nude mice xenografted with L-428 cells.
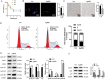
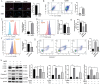
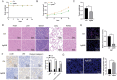
Results: We observed increased proliferation and autophagy in primary lymphoma tissues of patients. Administration of Ag85B inhibited the proliferation and autophagy of HL cell lines. Moreover, Ag85B promoted apoptotic pathway activation in vitro, which might be associated with mitochondrial dysfunction. Mechanistically, Ag85B inhibits autophagy by activating the phosphatidylinositol-4,5-bisphosphate 3-kinase/protein kinase B/mechanistic target of rapamycin kinase (PI3K/AKT/mTOR) and mitogen-activated protein kinase (MAPK) pathways. Ag85B also inhibited lymphoma growth in mice xenografted with HL cell lines, but no potential toxicity was observed.
Conclusion: Altogether, these results suggest that Ag85B inhibits HL growth via autophagy regulation. Current treatments for HL are associated with adverse events; therefore, Ag85B-mediated autophagy inhibition might be a promising strategy in to treat HL.
Keywords: Autophagy; Hodgkin disease; Mitogen-activated protein kinases; Mycobacterial antigen; Protein kinase B.
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest to report regarding the present study.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
