A review on pathobiology of circulating tumour plasma cells: The sine qua non of poor prognosis in plasma cell neoplasms
- PMID: 40296907
- PMCID: PMC12034004
- DOI: 10.32604/or.2024.055154
A review on pathobiology of circulating tumour plasma cells: The sine qua non of poor prognosis in plasma cell neoplasms
Abstract
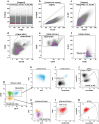
Circulating plasma cells (CPCs) in patients of plasma cell neoplasm have been an area of intense research in recent decades. Circulating tumor plasma cells (CTPCs) might represent a sub-clone of tumor cells that have exited into peripheral blood as a result of the dynamic interactions between the bone marrow (BM) microenvironment and neoplastic plasma cells. Chemokine receptors like chemokine receptor 4 (CXCR4) and integrins are known to play a role in homing and migration of plasma cells (PCs). The hypoxic microenvironment in the BM niche also contributes to their circulation through various mechanisms. In addition, the CCL3-CCR1 axis probably competes with the retention signals from the CXCR4-α4β1 (VLA-4) interaction and actively promotes the exit of PCs from the BM. CTPCs, even in extremely low numbers, can be detected and quantified by high-sensitivity techniques like multi-color flow cytometry and next-generation sequencing. High load of CTPCs noted in patients of plasma cell neoplasm; monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM), multiple myeloma (MM) is a strong predictor of shorter progression free survival (PFS) as well as overall survival (OS). In newly diagnosed patients of MM, a load of CTPCs correlates with the outcomes, i.e., OS and PFS. With more studies collaborating on the results of previous reports, assessment of the burden of CTPCs may become a complimentary approach for non-invasive risk stratification of MM patients and evaluating the response to therapy. Future research on larger cohorts and longer follow-ups may help to improve the existing staging system by incorporating the load of CTPCs as one of the prognostic indicators. Further studies based on isolation and genetic characterization of CTPCs may help in understanding the pathophysiology of the progression of the disease and may open avenues for newer treatment modalities. This review discusses the pathobiological aspects leading to circulation of neoplastic/tumor plasma cells in peripheral blood and provides a summary of research work done in last two decades on its prognostic importance in various plasma cells neoplasms.
Keywords: Circulating Plasma Cells (CPCs); Circulating Tumor Plasma Cells (CTPCs); Flow cytometry; Microenvironment; Monoclonal gammopathy; Multiple Myeloma (MM).
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest to report regarding the present study.
Figures

Similar articles
-
Detection of circulating normal and tumor plasma cells in newly diagnosed patients of multiple myeloma and their associations with clinical and laboratory parameters.Curr Probl Cancer. 2024 Feb;48:101025. doi: 10.1016/j.currproblcancer.2023.101025. Epub 2023 Nov 10. Curr Probl Cancer. 2024. PMID: 37951052
-
Next generation flow for minimally-invasive blood characterization of MGUS and multiple myeloma at diagnosis based on circulating tumor plasma cells (CTPC).Blood Cancer J. 2018 Nov 19;8(12):117. doi: 10.1038/s41408-018-0153-9. Blood Cancer J. 2018. PMID: 30455467 Free PMC article.
-
Enumeration and characterization of circulating multiple myeloma cells in patients with plasma cell disorders.Br J Haematol. 2018 Jan;180(1):71-81. doi: 10.1111/bjh.15003. Epub 2017 Nov 5. Br J Haematol. 2018. PMID: 29105742
-
Circulating plasma cells as a predictive biomarker in Multiple myeloma: an updated systematic review and meta-analysis.Ann Med. 2024 Dec;56(1):2338604. doi: 10.1080/07853890.2024.2338604. Epub 2024 Apr 10. Ann Med. 2024. PMID: 38599340 Free PMC article.
-
Monoclonal gammopathy and smoldering multiple myeloma: diagnosis, staging, prognosis, management.Recent Results Cancer Res. 2011;183:113-31. doi: 10.1007/978-3-540-85772-3_6. Recent Results Cancer Res. 2011. PMID: 21509683 Review.
References
-
- Kamili A, Ahmadi P, Leypoldt L, Marquard F, Schaefers C, Kosch R, et al. . Multiple myeloma in the young: insights on prognosis, clinical features and treatment outcome derived from nationwide German registry data and a nested multicenter sample. Haematologica. 2024 Nov 1;109(11):3795–9. - PMC - PubMed
-
- Soni S, Malhotra H. Outcome in patients with multiple myeloma: does age matter? Cancer Res Stat Treat. 2023 Sep;6(3):446. Tertiary cancer center. Ann Hematol. 2015 Jan;94(1):97–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
