4-(1-Methylamino)ethylidene-1,5-disubstituted pyrrolidine-2,3-diones: synthesis, anti-inflammatory effect and in silico approaches
- PMID: 40297251
- PMCID: PMC12035876
- DOI: 10.3762/bjoc.21.65
4-(1-Methylamino)ethylidene-1,5-disubstituted pyrrolidine-2,3-diones: synthesis, anti-inflammatory effect and in silico approaches
Abstract
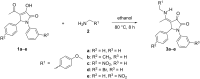
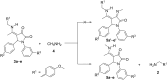
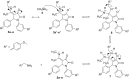
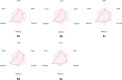
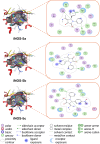
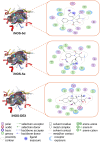
Pyrrolidine-2,3-diones are important intermediates in the synthesis of numerous nitrogen-containing heterocycles which possess a broad spectrum of biological and pharmacological activities. In this article, we report the synthesis of 4-(1-methylamino)ethylidene-1,5-disubstituted pyrrolidine-2,3-diones via a reversible transimination reaction between Schiff' base (C=N) linkage-containing pyrrolidine-2,3-dione derivatives and methylamine with yields of 80 to 92%. In addition to nuclear magnetic resonance spectroscopy, the structure of 4-(1-methylamino)ethylidene-1,5-diphenylpyrrolidine-2,3-dione (5a) was also verified through single-crystal X-ray diffraction. Furthermore, the synthesized molecules were evaluated for compliance with established drug-likeness rules (Lipinski, Veber, Ghose, Egan, and Muegge), as well as ADMET properties. All compounds satisfied these criteria, indicating favorable oral bioavailability. Molecular docking analysis showed that compounds 5a-e act as ligands for inducible nitric oxide synthase (iNOS), especially with Cys200 and Ser242 via hydrogen bonds. In addition, van der Waals interactions also contribute to the stabilization of the ligand-iNOS complexes. In particular, 4-(1-methylamino)ethylidene-5-phenyl-1-(3-nitrophenyl)pyrrolidine-2,3-dione (5e) exhibited the strongest binding affinity (-9.51 kcal/mol) and demonstrated significant inhibitory activity against nitric oxide (NO) production, with an IC50 value of 43.69 ± 5.26 µM. The presence of an electron-withdrawing group (-NO2 group) on the benzene ring at the 1-position of the pyrrolidine-2,3-dione subunit in compound 5e may be responsible for the observed high inhibition activity due to the enhancement and optimization of hydrogen bonding with Cys200. These results underscore the potential of 4-(1-methylamino)ethylidenepyrrolidine-2,3-diones, especially compound 5e, as promising scaffolds for the development of anti-inflammatory agents targeting iNOS-related pathologies.
Keywords: anti-inflammatory pyrrolidine-2,3-dione derivatives; iNOS; pyrrolidine-2,3-dione derivatives; pyrrolidine-2,3-diones; pyrrolidine-2,3-diones targeting reversible transimination reaction.
Copyright © 2025, Nguyen et al.
Figures
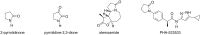

References
LinkOut - more resources
Full Text Sources
