Optimal Effect-Site Concentration of Propofol for Hemodynamic Stability During Intubation with Dexmedetomidine: A Randomized Controlled Study
- PMID: 40297310
- PMCID: PMC12036621
- DOI: 10.2147/DDDT.S508736
Optimal Effect-Site Concentration of Propofol for Hemodynamic Stability During Intubation with Dexmedetomidine: A Randomized Controlled Study
Abstract
Background: This study aimed to determine the 95% effective concentration (EC95) of propofol via target-controlled infusion (TCI) for endotracheal intubation at three different doses of dexmedetomidine.
Methods: One hundred and eighty patients aged 18-60 and classified as American Society of Anesthesiologists (ASA) class I-II were enrolled to undergo general anesthesia. Patients were randomly assigned to one of the three groups (A, B, or C), receiving three different doses of dexmedetomidine (0.6, 0.8, or 1 μg/kg) infused over 10 min. Anesthesia was then induced with propofol TCI, followed by rocuronium. The biased coin design method was used to calculate the EC95 of propofol for successful intubation. The primary outcome endpoint was the EC95 of propofol for successful endotracheal intubation at each dexmedetomidine dose.
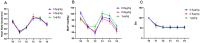
Results: Sixty patients in each group completed the trial. The time from propofol administration to intubation in group C (132.5 ± 10.7 s) was significantly shorter compared to group A (140.2 ± 14.4 s, P<0.0001) and group B (142.6 ± 13.2 s, P=0.0037). Both the EC95 and the average total dose of propofol in group B [14.6 (10.8, 14.8) μg/mL and 3.6 ± 1.1 mg/kg] and C [12.7 (11.5, 12.8) μg/mL and 2.8 ± 1.0 mg/kg] were lower than those in group A [14.9 (4.5, 15.0) μg/mL and 3.8 ± 0.9 mg/kg] (P<0.001). The incidence of hypotension and bradycardia during induction was low in each group.
Conclusion: The EC95 of propofol for endotracheal intubation across three different background doses of dexmedetomidine was determined. We suggest administering 1.0 μg/kg dexmedetomidine and then the EC95 of propofol for successful endotracheal intubation was 12.7 μg/mL.
Registration: Chinese Clinical Trial Registry; Registration number: ChiCTR2400089952, URL:https://www.chictr.org.cn/showproj.html?proj=221236.
Keywords: dexmedetomidine; effective concentration; endotracheal intubation; opioid-free anesthesia; propofol.
© 2025 Gao et al.
Conflict of interest statement
The authors disclose no conflicts of interest with respect to this work.
Figures

References
-
- Olausson A, Svensson CJ, Andrell P, Jildenstal P, Thorn SE, Wolf A. Total opioid-free general anaesthesia can improve postoperative outcomes after surgery, without evidence of adverse effects on patient safety and pain management: a systematic review and meta-analysis. Acta Anaesthesiol Scand. 2022;66(2):170–185. doi:10.1111/aas.13994 - DOI - PubMed
-
- Ao Y, Ma J, Zheng X, Zeng J, Wei K. Opioid-sparing anesthesia versus opioid-free anesthesia for the prevention of postoperative nausea and vomiting after laparoscopic bariatric surgery: a systematic review and network meta-analysis. Anesth Analg. 2024;140:385–396. doi:10.1213/ANE.0000000000006942 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

