Utilizing Physiologically Based Pharmacokinetic Models to Support Rational Medication in Chinese Elderly Population
- PMID: 40297312
- PMCID: PMC12034845
- DOI: 10.2147/DDDT.S501143
Utilizing Physiologically Based Pharmacokinetic Models to Support Rational Medication in Chinese Elderly Population
Abstract
Background: China is undergoing a pronounced shift towards an aging society, wherein the elderly constitute a prominent demographic relying significantly on medications. The imperative of administering rational medication to the elderly has gained considerable importance and warrants focused attention. The availability of pharmacokinetic (PK) data specific to the elderly is paramount for supporting informed medication practices. Unfortunately, studies addressing PK in the elderly are both infrequent and intricate, contributing to a lack of crucial data essential for tailoring personalized and rational medication approaches.
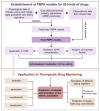
Methods: This study aimed to address this deficiency by employing the Physiologically Based Pharmacokinetic (PBPK) model, with the goal of supplying critical data to support rational medication strategies for the elderly. Additionally, we extended the application of PBPK models to Therapeutic Drug Monitoring (TDM) through the examination of four neuropsychiatric drugs.
Results: The PBPK models for 50 drugs in young and middle-aged Chinese adults were validated using clinical trial data. Simulated concentration-time curves closely matched the observed data, with Cmax and AUC ratios within 0.5-2.0. For Chinese elderly, PBPK models for four drugs (ticagrelor, rivaroxaban, alprazolam, midazolam) showed strong agreement with observed data. Comparing PK profiles of 50 drugs, no significant differences were found between elderly and younger adults. Dosage recommendations for four neuropsychiatric drugs in the elderly were provided based on simulation results, ensuring therapeutic effectiveness and safety.
Conclusion: In conclusion, PBPK models for 50 commonly prescribed drugs within the Chinese elderly population were developed, tackling general data gaps associated with these specific medications. Medication plans were developed specifically tailored for the elderly population, presenting an alternative methodology and perspective for the implementation of individualized and rational medication practices.
Keywords: PBPK model; individualized medicine; pharmacokinetics; rational medication; the elderly.
© 2025 Wu et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
-
- Statistics NBo. Bulletin of the seventh National Census. Available from: http://www.stats.gov.cn/xxgk/sjfb/zxfb2020/202105/t20210511_1817200.html. Accessed May 1, 2022.
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources

