Model-Informed Precision Dosing of Levamlodipine Besylate in Smoking Patients
- PMID: 40297316
- PMCID: PMC12036611
- DOI: 10.2147/DDDT.S501762
Model-Informed Precision Dosing of Levamlodipine Besylate in Smoking Patients
Abstract
Object: To quantitatively investigate the influence of various factors, including nicotine, demographics, biochemical index, and genetic polymorphisms of PAHs and drug metabolising enzymes, on the steady-state trough concentrations of levamlodipine besylate and its therapeutic effects in smokers. Using models to promote rational and accurate medication dosing in smoking patients when administered as initial monotherapy.
Methods: A prospective study (NCT05126381) enrolled 43 patients receiving levamlodipine monotherapy. Pop PK/PD model of levamlodipine besylate was established to investigate the effects of nicotine concentration, demographics (age, sex, height, weight, BMI), biochemical index (ALT, AST, ALB, UA, eGFR), and the genetic polymorphisms (rs4646903, rs1048943, rs762551, rs12459249, rs776746, rs2740574) on the patients' steady state trough concentrations and the antihypertensive efficacy (ΔSBP) of levamlodipine besylate after dosing.
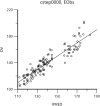
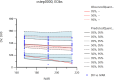
Results: The Pop PK/PD model was conducted using the study data of 43 patients. One-compartment model was used to describe the PK characteristics, and the direct effect model was used to describe the antihypertensive effect of levamlodipine besylate. The final Pop PK/PD model showed that the typical value of V = 3521L, CL = 62.6L·h-1, E0 = 168mmHg, Imax = 31mmHg, IC50 = 1.71ng·mL-1; eGFR and UA were found in the model had significant effect on the CL of levamlodipine besylate.
Conclusion: Patients with lower eGFR and UA levels exhibited lower CL levels, higher dosages may be considered for initial monotherapy in such patients. The current study tentatively do not show that nicotine concentration and PAHs metabolizing enzymes have significant effect on PK and PD in patients taking the drug. More data may be needed in the future to refine the effects of the above covariates on the PK and PD parameters of the levamlodipine besylate.
Keywords: Levamlodipine besylate; model-informed precision dosing; pop PK/PD model; smoking patients.
© 2025 Li et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures






Similar articles
-
Effects of antihypertensive drugs losartan and levamlodipine besylate on insulin resistance in patients with essential hypertension combined with isolated impaired fasting glucose.Hypertens Res. 2016 May;39(5):321-6. doi: 10.1038/hr.2015.155. Epub 2016 Jan 14. Hypertens Res. 2016. PMID: 26763851 Clinical Trial.
-
Effect of low-dose Levamlodipine Besylate in the treatment of vascular dementia.Sci Rep. 2019 Dec 3;9(1):18248. doi: 10.1038/s41598-019-47868-0. Sci Rep. 2019. PMID: 31796756 Free PMC article.
-
Randomized, two-way crossover bioequivalence study of levamlodipine besylate tablets in healthy Chinese subjects .Int J Clin Pharmacol Ther. 2017 Oct;55(10):818-824. doi: 10.5414/CP202998. Int J Clin Pharmacol Ther. 2017. PMID: 28619129 Clinical Trial.
-
Combatting the Rising Tide of Antimicrobial Resistance: Pharmacokinetic/Pharmacodynamic Dosing Strategies for Maximal Precision.Int J Antimicrob Agents. 2021 Mar;57(3):106269. doi: 10.1016/j.ijantimicag.2020.106269. Epub 2020 Dec 23. Int J Antimicrob Agents. 2021. PMID: 33358761 Review.
-
The role of population PK-PD modelling in paediatric clinical research.Eur J Clin Pharmacol. 2011 May;67 Suppl 1(Suppl 1):5-16. doi: 10.1007/s00228-009-0782-9. Epub 2010 Mar 26. Eur J Clin Pharmacol. 2011. PMID: 20340012 Free PMC article. Review.
References
-
- FDA. FDA approved drug products: conjupri Levamlodipine oral tablets. 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212895s000lbl.pdf. Accessed April 19, 2025.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

