Complement inhibitor therapy in thymoma-associated myasthenia gravis: a real-world experience
- PMID: 40297583
- PMCID: PMC12034619
- DOI: 10.3389/fimmu.2025.1562419
Complement inhibitor therapy in thymoma-associated myasthenia gravis: a real-world experience
Abstract
Introduction: Thymoma-associated myasthenia gravis (TAMG) accounts for 15-20% of all myasthenia gravis (MG) cases and is typically characterized by severe clinical manifestations and suboptimal response to conventional therapies. However, TAMG patients are underrepresented in clinical trials, leaving gaps in evidence for optimal treatment strategies. This study assessed the efficacy of complement inhibitors (CI) in TAMG population.
Methods: We retrospectively reviewed 23 TAMG patients who received CI, with a minimum follow-up of six months. Additionally, we randomly included 22 MG patients without thymoma, treated with CI, in the control group. Clinical outcomes were measured using Myasthenia Gravis-Activities of Daily Living (MG-ADL) and Quantitative Myasthenia Gravis (QMG) scores at baseline, three, and six months.
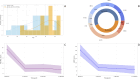
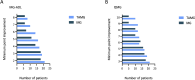
Results: Among the 23 TAMG patients, 21 initiated CI after thymectomy, with a median interval of eight years (IQR:2.5-15) post-surgery. Two patients achieved sufficient stabilization on CI to undergo thymectomy thereafter. The most frequent thymoma histological subtype was WHO type B2, detected in 43.5% of cases. Median MG-ADL score decreased from 11 (IQR:8-15) to 3 (IQR:2-5) and 4 (IQR:1-5) at three and six months, respectively (both p<0.001). Median QMG score decreased from 16 (IQR:14-22) to 10 (IQR: 5-11) at three and six months (both p<0.001). Prednisone dosage was tapered in 20 patients. No significant differences were observed between TAMG and MG patients without thymoma in MG-ADL, QMG and steroid reduction.
Conclusion: CI demonstrated significant improvements in MG-ADL and QMG scores, along with a steroid-sparing effect, suggesting its potential as an effective treatment for this challenging subpopulation.
Keywords: complement inhibitors therapy; myasthenia gravis; neuroimmunology; neuromuscular disease; real-world; thymoma.
Copyright © 2025 Marini, Erra, Fionda, Falso, Rossini, Habetswallner, Meacci, Marini, Habetswallner and Iorio.
Conflict of interest statement
RI has received consultancy fees and speaker honoraria from Alexion, Argenx, UCB and Dianthus Therapeutics. LF served as a consultant/advisor for Alexion, Argenx, UCB and Dianthus. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

