The mechanisms of HER2 targeted ADCs are dependent on Rab GTPases
- PMID: 40297622
- PMCID: PMC12035104
- DOI: 10.1177/17588359251332473
The mechanisms of HER2 targeted ADCs are dependent on Rab GTPases
Abstract
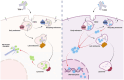
Introduction: In the era of personalized cancer therapy, antibody-drug conjugates (ADCs) have become one of the fastest-emerging groups of anticancer drugs. ADCs consist of an antibody coupled to a cytotoxic payload by a chemical linker, designed to be cleaved off intracellularly. Understanding the intracellular trafficking and processing of ADCs is crucial for elucidating their mechanism of action.
Objective: This study aimed to compare trastuzumab deruxtecan (T-DXd) to ado-trastuzumab emtansine (T-DM1) with emphasis on Rab GTPase-regulated intracellular trafficking and its impact on ADC efficacy.
Methods: The efficacy of T-DXd and T-DM1 was assessed in a panel of HER2-positive cell lines. Correlations between ADC efficacy and the expression of HER2 and Rab GTPases were evaluated. Functional studies, including knockdown (KD), overexpression, and microscopy, were performed to evaluate the impact of Rab GTPases on ADC cytotoxicity.
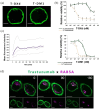
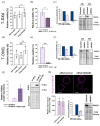
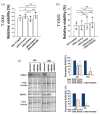
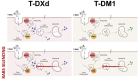
Results: In contrast to T-DM1, T-DXd efficacy was found not to correlate to HER2 expression in a panel of HER2-positive cell lines. However, a correlation to RAB5A expression was found for T-DXd efficacy, although not as strong as for T-DM1. Altering the expression of RAB5 in our model system confirmed RAB5 to have an impact on both T-DXd and T-DM1 cytotoxicity, but more on T-DM1. In addition, RAB4a was found to influence T-DXd sensitivity, but not T-DM1, indicating differences in intracellular processing between T-DXd and T-DM1.
Conclusion: The study demonstrates that ADC design significantly influences intracellular trafficking and processing. The linker design, in particular, plays a major role in determining the intracellular fate of an ADC.
Keywords: ADC; HER2; RAB5; T-DM1; T-DXd; breast cancer; mechanism of action.
© The Author(s), 2025.
Conflict of interest statement
A patent application entitled “Diagnosis and treatment of cancer” by A.W., K.B., O.E., and Maria E.B. Berstad, application number: WO 2018/234872 A1 is currently in the national phase. Also, A.W., O.E., and K.B. are co-founders of Rab Diagnostics, a Norwegian biotech startup developing predictive biomarkers for ADCs. AW is also CEO in Rab Diagnostics.
Figures


References
-
- Dumontet C, Reichert JM, Senter PD, et al.. Antibody–drug conjugates come of age in oncology. Nat Rev Drug Discov 2023; 22(8): 641–661. - PubMed
-
- Zolot RS, Basu S, Million RP. Antibody–drug conjugates. Nat Rev Drug Discov 2013; 12(4): 259–260. - PubMed
-
- Khongorzul P, Ling CJ, Khan FU, et al.. Antibody–drug conjugates: a comprehensive review. Mol Cancer Res 2020; 18(1): 3–19. - PubMed
-
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2001; 2(2): 107–117. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

