Lipotoxicity: A New Perspective in Type 2 Diabetes Mellitus
- PMID: 40297768
- PMCID: PMC12036605
- DOI: 10.2147/DMSO.S511436
Lipotoxicity: A New Perspective in Type 2 Diabetes Mellitus
Abstract
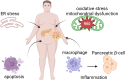
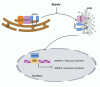
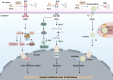
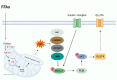
Type 2 diabetes mellitus is a non-communicable metabolic disorder characterized by insulin resistance (IR) associated with defects in insulin production and secretion. Recent studies have shown that lipotoxicity, which is characterized by the abnormal accumulation of lipids in non-adipose tissues, leads to bodily dysfunction and metabolic disorders, thereby promoting the progression of T2DM. This process is mediated by the induction of endoplasmic reticulum (ER) stress, oxidative stress (OS), mitochondrial dysfunction, and inflammatory responses in pancreatic β-cells, ultimately leading to the activation of apoptosis pathways, which results in β-cell dysfunction and cell death. Furthermore, lipotoxicity interferes with insulin signaling pathways, which worsens IR. Current clinical approaches aimed at mitigating lipotoxicity-induced IR and β-cell dysfunction include the use of metformin, glucagon-like peptide-1 analogs, thiazolidinediones, and molecular chaperones, in addition to interventions such as caloric restriction and physical activity, which reduce fat accumulation in the pancreas and enhance β-cell function. Investigating the interplay between lipotoxicity and T2DM is essential for understanding the underlying disease mechanisms and providing new insights into prevention and therapeutic strategies. This review offers a comprehensive analysis of the mechanisms underlying lipotoxicity in T2DM, highlighting how these insights may drive future research and inform the development of novel treatment approaches.
Keywords: adipose tissue; insulin resistance; lipotoxicity; type 2 diabetes mellitus.
© 2025 Chen et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
Molecular mechanisms of lipotoxicity-induced pancreatic β-cell dysfunction.Int Rev Cell Mol Biol. 2021;359:357-402. doi: 10.1016/bs.ircmb.2021.02.013. Epub 2021 Mar 12. Int Rev Cell Mol Biol. 2021. PMID: 33832653 Review.
-
Insulin Resistance, Obesity and Lipotoxicity.Adv Exp Med Biol. 2017;960:277-304. doi: 10.1007/978-3-319-48382-5_12. Adv Exp Med Biol. 2017. PMID: 28585204 Review.
-
Pancreatic Fat is not significantly correlated with β-cell Dysfunction in Patients with new-onset Type 2 Diabetes Mellitus using quantitative Computed Tomography.Int J Med Sci. 2020 Jul 2;17(12):1673-1682. doi: 10.7150/ijms.46395. eCollection 2020. Int J Med Sci. 2020. PMID: 32714070 Free PMC article.
-
Polydatin prevents lipotoxicity-induced dysfunction in pancreatic β-cells by inhibiting endoplasmic reticulum stress and excessive autophagy.Phytomedicine. 2022 Nov;106:154410. doi: 10.1016/j.phymed.2022.154410. Epub 2022 Aug 20. Phytomedicine. 2022. PMID: 36030747
Cited by
-
p47phox: A Central Regulator of NADPH Oxidase Function and a Promising Therapeutic Target in Redox-Related Diseases.Cells. 2025 Jul 8;14(14):1043. doi: 10.3390/cells14141043. Cells. 2025. PMID: 40710296 Free PMC article. Review.
References
-
- Ong KL, Stafford LK, McLaughlin SA, et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203–234. doi:10.1016/s0140-6736(23)01301-6 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

