Plasma Levels of CXCL9 and MCP-3 are Increased in Asthma-COPD Overlap (ACO) Patients
- PMID: 40297845
- PMCID: PMC12034844
- DOI: 10.2147/COPD.S506517
Plasma Levels of CXCL9 and MCP-3 are Increased in Asthma-COPD Overlap (ACO) Patients
Abstract
Purpose: Asthma and chronic obstructive pulmonary disease overlap patients (ACO) have more exacerbations and a worse prognosis than pure asthma or COPD, and it is of great interest to identify differential biomarkers of ACO. We compared blood eosinophil counts, plasma IgE and protein levels among patients with asthma, ACO, COPD, and healthy subjects to identify those associated with ACO.
Patients and methods: 397 adults (age 40-90 years) were recruited from two Colombian cities: asthma (n=123), COPD (n=100), ACO (n=74) and healthy control (HC, n=100). Plasma protein levels were measured using the Proximity Extension Assay (Olink Proteomics).
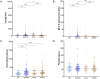
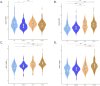
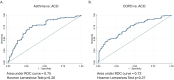
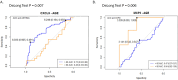
Results: There were no differences in blood eosinophil counts between the patient groups. Total and specified IgE levels were higher in patients with ACO than in those with COPD. Ten plasma proteins showed significant differences between the patients with ACO and HC. In patients above 60 years old, CXCL9 discriminates ACO from asthma patients with AUC 0.73 (0.63-0.82, DeLong test p=0.007), and in patients below 60 years old, MCP-3 discriminates ACO from COPD patients with AUC 0.84 (0.62-1.0, DeLong test p=0.006). CUB domain-containing protein 1 (CDCP1) levels (OR, 0.47; p=0.008) and age > 60 years (OR, 0.25; p=0.001) were negatively associated with ACO.
Conclusion: CXCL9 levels could be used to discriminate ACO from asthma patients and MCP-3 to discriminate ACO from COPD. Protein inflammatory signatures in plasma of ACO patients were similar to the COPD group. This study revealed novel biomarkers that may help characterize patients with ACO.
Keywords: COPD; CXCL9; MCP-3; PEA; biomarkers; chronic obstructive pulmonary disease; circulating inflammatory mediators; proximity extension assay.
© 2025 Escamilla-Gil et al.
Conflict of interest statement
Dr Kevin Llinás-Caballero reports grants from Ministry of Science of the Republic of Colombia, grants from University of Cartagena, during the conduct of the study. Dr Nathalie Acevedo reports grants from Ministry of Science, Republic of Colombia, during the conduct of the study. The author(s) report no conflicts of interest in this work.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

