Outbreak of Enterovirus D68 in Young Children, Brescia, Italy, August to November 2024
- PMID: 40297997
- PMCID: PMC12038777
- DOI: 10.1002/jmv.70372
Outbreak of Enterovirus D68 in Young Children, Brescia, Italy, August to November 2024
Abstract
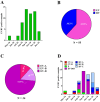
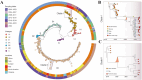
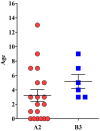
Enterovirus D68 (EV-D68) is responsible for a plethora of clinical manifestations ranging from asymptomatic infections to severe respiratory symptoms and neurological disorders. EV-D68 was first detected in children with pneumonia in 1962 and, from then, only sporadic cases were reported until 2014, when outbreaks were notified across the world. After the withdrawal of preventive measures against SARS-CoV-2, a significant increase in EV-D68 infections has been reported in 2021-2022. A surveillance program to evaluate the incidence of enterovirus/rhinovirus (EV/RV) infections was implemented at the Brescia Civic Hospital, Italy. Fifty-five EV/RV-positive respiratory samples, belonging to pediatric patients, were subjected to NGS. We observed that 61.8% of samples were positive for EV, with EV-D68 as the most prevalent genotype predominantly detected between August and November 2024. Phylogenetic analysis revealed that EV-D68 sequences formed two monophyletic clades corresponding to the A2 and B3 lineages, highlighting their recent introduction in Italy. Interestingly, 40% of pediatric EV-D68 infections were detected with at least one other EV/RV. Our study highlights the crucial role played by genomic surveillance of respiratory infections to monitor the circulation of emerging and re-emerging viruses, as well as their evolution. This will be fundamental to enable prompt intervention strategies.
Keywords: children; clinical presentation; enterovirus D68; epidemiology; phylogenetic analysis.
© 2025 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Midgley S. E., Benschop K., Dyrdak R., et al., “Co‐Circulation of Multiple Enterovirus D68 Subclades, Including a Novel B3 Cluster, Across Europe in a Season of Expected Low Prevalence, 2019/20,” Eurosurveillance 25, no. 2 (2020): 1900749, 10.2807/1560-7917.ES.2020.25.2.1900749. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

