Disruption of DNA methylation underpins the neuroinflammation induced by targeted CNS radiotherapy
- PMID: 40298030
- PMCID: PMC12404709
- DOI: 10.1093/brain/awaf163
Disruption of DNA methylation underpins the neuroinflammation induced by targeted CNS radiotherapy
Abstract
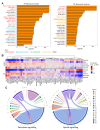
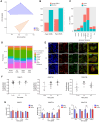
Targeted radiotherapy is integral to the increasing survival of cancer patients; however, it has significant side effects, the underlying cellular and molecular mechanisms of which are ill-defined. It is well documented that targeted radiotherapy induces epigenetic changes in neoplastic tissue, which impacts tumour evolution; however, whether epigenetic deregulation also occurs in the surrounding non-neoplastic tissue and contributes to the occurrence of side effects is unknown. We characterized the DNA methylome in a unique cohort of irradiated peri-lesional brain tissue samples and integrated it with gene expression analysis at the spatial level. We show differences in DNA methylation patterns in irradiated brain tissue and identify specific inflammatory micro-environmental niches and their regulatory neuropeptides after irradiation. Finally, we show in a cerebral organoid model, that the same neuropeptides are upregulated as well as similar DNA methylation alterations and disruption of the DNA methylation machinery, in keeping with the interpretation that epigenetic dysregulation plays a role in neurotoxicity, hence raising the possibility it could represent a novel target for the reduction of radiotherapy side effects.
Keywords: DNA methylation; epigenetics; neuro-oncology; neuroinflammation; radiotherapy; spatial transcriptomics.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures




Comment in
-
Tackling neuronal stress and neuroinflammation to prevent side effects of brain radiotherapy.Brain. 2025 Sep 3;148(9):3026-3028. doi: 10.1093/brain/awaf270. Brain. 2025. PMID: 40696973 No abstract available.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

