Case Series of Canine Myasthenia Gravis: A Classification Approach With Consideration of Seronegative Dogs
- PMID: 40298067
- PMCID: PMC12038433
- DOI: 10.1111/jvim.70113
Case Series of Canine Myasthenia Gravis: A Classification Approach With Consideration of Seronegative Dogs
Abstract
Background: Myasthenia gravis (MG) is categorized into several subgroups, including seronegative MG. Seronegative human patients are well documented, but seronegative dogs remain clinically uncharacterized and their prevalence unknown.
Objectives: This study aims to evaluate the clinical presentation, diagnosis, treatment, and outcome of canine MG subgroups.
Animals: One hundred sixty-seven owner-owned dogs diagnosed with MG from three referral centers.
Methods: Retrospective case series. We classified myasthenic dogs into subgroups, adhering to human guidelines.
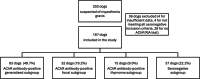
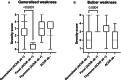
Results: We classified 167 dogs into four subgroups: acetylcholine receptor (AChR) antibody-positive generalized (49.7%, n = 83/167), focal (19.2%, n = 32/167) and thymoma-associated MG (9%, n = 15/167) and seronegative MG (22.2%, n = 37/167). Dogs with thymoma-associated MG were older (median 102 months; Interquartile Range (IQR) 96-120; p < 0.001) and seronegative dogs were younger (median 30 months; IQR 11.5-66; p = 0.017), compared to the generalized subgroup (median 67 months; IQR 36-96). Seronegative dogs presented less frequently with megaesophagus, compared to the generalized subgroup (63.8% vs. 85.7%; Odds Ratio 3.4; 95% confidence intervals (C.I.) 1.4-8.9; p = 0.025). Myasthenic dogs' survival time was significantly reduced when thymoma (Hazard Ratio (H.R.) 3.7; 95% C.I. 1.4-9.9; p = 0.028) or esophageal weakness (H.R. 3.8; 95% C.I. 2.0-7.0; p < 0.001) was present. Conversely, a higher likelihood of remission was achieved when esophageal weakness was absent (H.R. 3.8; 95% C.I. 1.4-10.0; p = 0.007).
Conclusion and clinical importance: Dogs with seronegative MG are more common than previously reported. Myasthenic subgroups differ in presentation and outcome, with esophageal weakness key to survival and remission. Diagnostic tests for seronegative dogs and effective treatments for esophageal weakness in myasthenic dogs are urgently needed.
Keywords: acetylcholine receptor; antibody test; autoimmune; junctionopathy.
© 2025 The Author(s). Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Gilhus N. E., Tzartos S., Evoli A., Palace J., Burns T. M., and Verschuuren J. J. G. M., “Myasthenia Gravis,” Nature Reviews Disease Primers 5, no. 30 (2019). - PubMed
-
- Gilhus N. E. and Verschuuren J. J., “Myasthenia Gravis: Subgroup Classification and Therapeutic Strategies,” Lancet Neurology 14 (2015): 1023–1036. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

