Treatment Efficacy and Molecular Dynamics of Neoadjuvant Durvalumab and Olaparib in Resectable Urothelial Bladder Cancer: The NEODURVARIB Trial
- PMID: 40298406
- PMCID: PMC12010967
- DOI: 10.1158/1078-0432.CCR-24-2890
Treatment Efficacy and Molecular Dynamics of Neoadjuvant Durvalumab and Olaparib in Resectable Urothelial Bladder Cancer: The NEODURVARIB Trial
Abstract
Purpose: Neoadjuvant treatment of bladder cancer is evolving, with immunotherapy demonstrating promising activity. PARP inhibition combined with immune activation has been proposed as a synergistic strategy. We conducted a comprehensive molecular characterization of tumors treated with this combination in the neoadjuvant setting to provide crucial results for rational development.
Patients and methods: A phase II clinical trial was designed to evaluate the combination of anti-PDL1 inhibitor durvalumab and PARP inhibitor olaparib, focusing on biomarker dynamics in both pre- and post-treatment settings. A total of 29 patients were enrolled. Genomic and transcriptomic profiling, as well as analyses of immune cell populations, was conducted at baseline and at the time of cystectomy.
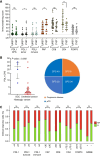
Results: Of the 29 patients treated, a pathologic complete response was observed in 13 cases (44.8%). No major safety concerns were associated with the treatment, and 26 patients (90%) underwent cystectomy. Mutational patterns, tumor mutation burden, and homologous recombination deficiency remained stable throughout treatment and were not predictive of outcomes. However, a shift toward stromal phenotypes and increased expression of epithelial-mesenchymal transition signatures were observed following therapy, particularly in resistant tumors. Moreover, an increase in circulating CD4+ CD27- CD28- T cells was noted among responders.
Conclusions: The combination of neoadjuvant durvalumab and olaparib shows therapeutic activity in bladder cancer. Resistance mechanisms seem to be driven by transcriptional adaptations rather than the emergence of new mutations.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
J.F. Rodríguez-Moreno reports grants, personal fees, and nonfinancial support from AstraZeneca during the conduct of the study, as well as grants, personal fees, and nonfinancial support from Bayer, Pierre Fabre, Regeneron, Bristol Myers Squibb, Novartis, Johnson & Johnson, and MSD, personal fees and nonfinancial support from Astellas and Pfizer, grants from Ipsen, and grants and personal fees from MERCK outside the submitted work, as well as employment at HM Hospitales and the Spanish Oncology Genito-Urinary Group. G. de Velasco reports personal fees from Astellas, Bristol Myers Squibb, Ipsen, AstraZeneca, Janssen, Bayer, MSD, Merck, Eisai, and Roche outside the submitted work. C. Álvarez-Fernández reports personal fees from Pfizer, Merck, Ipsen, Bayer, and AstraZeneca outside the submitted work. S. Vázquez reports grants from AstraZeneca during the conduct of the study, as well as grants from Bayer, Astellas, Bristol Myers Squibb, Ipsen, Roche, Janssen, and Pfizer outside the submitted work. P. Gajate reports other support from Bristol Myers Squibb, Merck, Roche, MSD, and Astellas outside the submitted work. A. Font reports grants from Roche and Astellas outside the submitted work. N. Lainez reports other support from AstraZeneca during the conduct of the study, as well as other support from Astellas, Bristol Myers Squibb, MSD, Merck, and Ipsen outside the submitted work. J. García-Donas reports grants from AstraZeneca during the conduct of the study, as well as grants and personal fees from Bristol Myers Squibb, AstraZeneca, Bayer, Astellas, Ipsen, Merck, MSD, Gilead, Novartis, Pharmamar, GlaxoSmithKline, Lilly, Pfizer, Roche, Janssen, and Piere Fabre outside the submitted work. No disclosures were reported by the other authors.
Figures



References
-
- Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. . Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66. - PubMed
-
- Powles T, Catto JWF, Galsky MD, Al-Ahmadie H, Meeks JJ, Nishiyama H, et al. . Perioperative durvalumab with neoadjuvant chemotherapy in operable bladder cancer. N Engl J Med 2024;391:1773–86. - PubMed
-
- Necchi A, Anichini A, Raggi D, Briganti A, Massa S, Lucianò R, et al. . Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol 2018;36:3353–60. - PubMed
-
- Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. . Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 2020;383:1218–30. - PubMed
-
- Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. . Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1547–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

