Role of glucuronoxylomannan and steryl glucosides in protecting against cryptococcosis
- PMID: 40298449
- PMCID: PMC12153286
- DOI: 10.1128/mbio.00984-25
Role of glucuronoxylomannan and steryl glucosides in protecting against cryptococcosis
Abstract
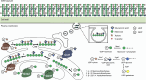
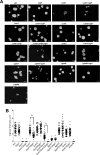
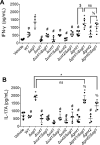
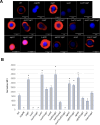
The development of vaccines for fungal diseases, including cryptococcosis, is an emergent line of research and development. In previous studies, we showed that a Cryptococcus mutant lacking the SGL1 gene (∆sgl1) accumulates certain glycolipids called steryl glucosides (SGs) on the fungal capsule, promoting an effective immunostimulation that totally protects the host from a secondary cryptococcal infection. However, this protection is lost when the cryptococcal capsule is absent in the ∆sgl1 background. The cryptococcal capsule is mainly composed of glucuronoxylomannan (GXM), a polysaccharide microfiber consisting of glucuronic acid, xylose, and mannose linked by glycosidic bonds forming specific triads. In this study, we engineered cells to lack each of the GXM components and tested the effect of these deletions on protection under the condition of SG accumulation. We found that glucuronic acid and xylose are required for protection, and their absence abrogates the production of IFNγ and IL-17A by γδ T cells, which are necessary stimulants for the protective phenotype of the ∆sgl1. We analyzed the structure of the GXM microfibers and found that although the deletion of SGL1 only slightly affects the size and distribution of these microfibers, it significantly changes the ratio of mannose to other components. In conclusion, this study identifies the structural modifications that the deletion of SGL1 and the consequent accumulation of SGs impart to the GXM structure of C. neoformans. This provides significant insights into the protective mechanisms mediated by SG accumulation on the capsule, with important implications for the future development of an efficacious cryptococcal vaccine.IMPORTANCECryptococcus neoformans is an encapsulated fungus that causes invasive fungal infections with high morbidity and mortality in susceptible patients. With increasing drug resistance and high toxicity of current antifungal drugs, there is a need for alternative therapeutic strategies, such as a cryptococcal vaccine. In this study, we identify the necessary capsular components and their structural organization required for a cryptococcal vaccine to protect the host against challenge with a virulent strain. These capsular components are glucuronic acid, xylose, and mannose, and they work together with certain glycolipids called steryl glucosides (SGs) to stimulate host immunity. Interestingly, SGs on the capsule may favor the formation of small capsular microfibers organized in specific mannose triads. Thus, the results of this paper are important because they identify a mechanism by which SGs affect the structure of the cryptococcal capsule, with important implications for the future development of a cryptococcal vaccine using capsular components and SGs.
Keywords: Cryptococcus neoformans; fungal infection; glucuronic acid; glucuronoxylomannan; immunity; mannose; steryl glucosides; vaccine; xylose.
Conflict of interest statement
Maurizio Del Poeta is a co-founder and the chief scientific officer of MicroRid Technologies Inc. All other authors declare no competing interests.
Figures




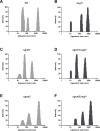
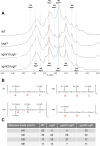
References
-
- Walukaga S, Fieberg A, Musubire A, Tugume L, Ssebambulidde K, Kagimu E, Kasibante J, Rutakingirwa MK, Mpoza E, Gakuru J, Akampurira A, Jjunju S, Mwesigye J, Muzoora C, Nuwagira E, Bangdiwala AS, Williams DA, Rhein J, Meya DB, Boulware DR, Hullsiek KH, Rajasingham R, ASTRO team . 2024. The evolution of HIV-associated cryptococcal meningitis in Uganda from 2010 to 2022. Med Mycol 63:myae115. doi: 10.1093/mmy/myae115 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- I01 BX002624/BX/BLRD VA/United States
- IK6 BX005386/BX/BLRD VA/United States
- AI184929/National Institute of Allergy and Infectious Diseases
- AI135012/National Institute of Allergy and Infectious Diseases
- AI125770/National Institute of Allergy and Infectious Diseases
- I01BX002624/U.S. Department of Veterans Affairs
- DMR-1933525/National Science Foundation
- R01 AI135012/AI/NIAID NIH HHS/United States
- DE-SC0015662/U.S. Department of Energy
- R01 AI184929/AI/NIAID NIH HHS/United States
- GM137782/GM/NIGMS NIH HHS/United States
- DMR-1905547/National Science Foundation
- R01 AI116420/AI/NIAID NIH HHS/United States
- R01 AI125770/AI/NIAID NIH HHS/United States
- AI116420/National Institute of Allergy and Infectious Diseases
- R24 GM137782/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
